Possible coronavirus drug identified by Australian scientists
- A collaborative study led by Monash University’s Biomedicine Discovery Institute (BDI) in Melbourne, Australia, with the Peter Doherty Institute of Infection and Immunity (Doherty Institute), has shown that an anti-parasitic drug already available around the world kills the virus within 48 hours.
- Australian Scientists have shown that an anti-parasitic drug already available around the world can kill the virus within 48 hours.
- Scientists from Monash University in Melbourne showed that a single dose of the drug, Ivermectin, could stop the SARS-CoV-2 virus growing in cell culture – effectively eradicating all genetic material of the virus within 48 hours.
- The next steps are to determine the correct human dosage – ensuring the doses shown to effectively treat the virus in the test tube are safe levels for humans.
- The use of Ivermectin to combat COVID-19 depends on pre-clinical testing and clinical trials, with funding urgently required to progress the work.
- Ivermectin is an FDA-approved anti-parasitic drug that has also been shown to be effective in vitro against a broad range of viruses including HIV, Dengue, Influenza and Zika virus.
- The findings of the study were published today in Antiviral Research.
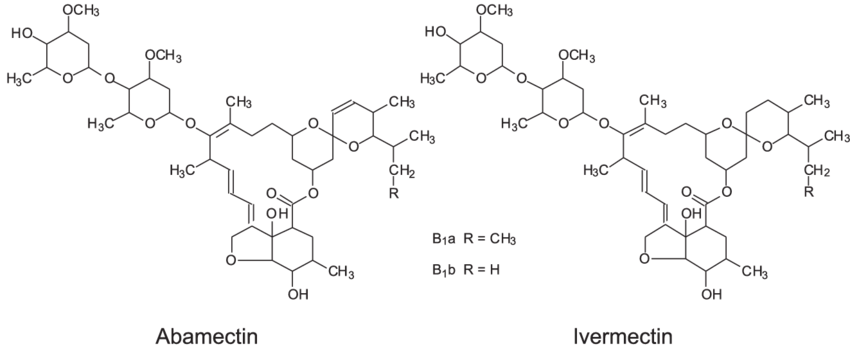
Structure-of-abamectin-and-ivermectin

Monash_ University_Biomedicine_Discovery_Institute

Peter_Doherty_Institute_of_Infection_and_Immunity
About Ivermectin:
Ivermectin is a medication used to treat many types of parasite infestations. This includes head lice, scabies, river blindness (onchocerciasis), strongyloidiasis, trichuriasis, ascariasis, and lymphatic filariasis. It can be taken by mouth or applied to the skin for external infestations. Use in the eyes should be avoided.
Ivermectin has also been found to work against HIV, influenza and Zika virus.
Common side effects include red eyes, dry skin, and burning skin. It is unclear if it is safe for use during pregnancy, but is probably acceptable for use during breastfeeding. It belongs to the avermectin family of medications. It works by causing the parasite’s cell membrane to increase in permeability, resulting in paralysis and death.
Ivermectin was discovered in 1975 and came into medical use in 1981. It is on the World Health Organization’s List of Essential Medicines, the safest and most effective medicines needed in a health system. The wholesale cost in the developing world for the tablets is about US$0.12 for a course of treatment. In the United States, the costs are less than US$50. In other animals, it is used to prevent and treat heartworm among other diseases.
Half of the 2015 Nobel Prize in Physiology or Medicine was awarded jointly to Campbell and Ōmura for discovering avermectin, “the derivatives of which have radically lowered the incidence of river blindness and lymphatic filariasis, as well as showing efficacy against an expanding number of other parasitic diseases.
CAS RN. of Ivermectin 70288-86-7; 71827-03-7. There are more than 100 brand names of Ivermectin in the market today some modern trade names are Stromectol, Soolantra cream.
Press Release by Monash University
A collaborative study led by Monash University’s Biomedicine Discovery Institute (BDI) in Melbourne, Australia, with the Peter Doherty Institute of Infection and Immunity (Doherty Institute), has shown that an anti-parasitic drug already available around the world kills the virus within 48 hours.
The Monash Biomedicine Discovery Institute’s Dr. Kylie Wagstaff, who led the study, said the scientists showed that the drug, Ivermectin, stopped the SARS-CoV-2 virus growing in cell culture within 48 hours.
“We found that even a single dose could essentially remove all viral RNA by 48 hours and that even at 24 hours there was a really significant reduction in it,” Dr. Wagstaff said.
Ivermectin is an FDA-approved anti-parasitic drug that has also been shown to be effective in vitro against a broad range of viruses including HIV, Dengue, Influenza and Zika virus.
Dr. Wagstaff cautioned that the tests conducted in the study were in vitro and that trials needed to be carried out in people.
“Ivermectin is very widely used and seen as a safe drug. We need to figure out now whether the dosage you can use it at in humans will be effective – that’s the next step,” Dr. Wagstaff said.
“In times when we’re having a global pandemic and there isn’t an approved treatment, if we had a compound that was already available around the world then that might help people sooner. Realistically it’s going to be a while before a vaccine is broadly available.
Although the mechanism by which Ivermectin works on the virus is not known, it is likely, based on its action in other viruses, that it works to stop the virus ‘dampening down’ the host cells’ ability to clear it, Dr. Wagstaff said.
Royal Melbourne Hospital’s Dr. Leon Caly, a Senior Medical Scientist at the Victorian Infectious Diseases Reference Laboratory (VIDRL) at the Doherty Institute where the experiments with live coronavirus were conducted, is the study’s first author.
“As the virologist who was part of the team who were first to isolate and share SARS-COV2 outside of China in January 2020, I am excited about the prospect of Ivermectin being used as a potential drug against COVID-19,” Dr. Caly said.
Dr. Wagstaff made a previous breakthrough finding on Ivermectin in 2012 when she identified the drug and its antiviral activity with Monash Biomedicine Discovery Institute’s Professor David Jans, also an author on this paper. Professor Jans and his team have been researching Ivermectin for more than 10 years with different viruses.
Dr. Wagstaff and Professor Jans started investigating whether it worked on the SARS-CoV-2 virus as soon as the pandemic was known to have started.
The use of Ivermectin to combat COVID-19 would depend on the results of further pre-clinical testing and ultimately clinical trials, with funding urgently required to keep progressing the work, Dr. Wagstaff said.
###
Read the full paper in Antiviral Research titled: The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro: https://www.sciencedirect.com/science/article/pii/S0166354220302011
To Find More details subscribe AgroPat: Visit http://chemrobotics.com
- AgroPat provides techno-legal solutions to Agrochemical Discovery and Generic industry. (https://www.chemrobotics.com/agropat-lighter/login)
- AgroPat platforms cover more than 4500 Active Ingredients (Al’s) with all information including synthesis (ROS), formulation, combination, innovator, patents including product patent, developer, utmost important biology data containing spectrum, MoA, DFU, toxicity, which can play a major role in decision making for any individual /company/ university/ industry.
- AgroPat Open Access – Free access to everyone,
- AgroPat Lite – With limited information as per business function needs,
- AgroPat ultimate – With all technical and business information,
- Provide aboriginal information, which enables product developers to know about the state of the art in relevant subject matter, which includes innovator Patent / Non-Patent literature, which is critical for the generic industry.
- Indian Pesticide Database (IPD) offers pesticide product registration/approval Information in various categories including Section 9(3), Section 9(3B), Section 9(4) and Bio-pesticides (https://www.chemrobotics.com/agropat/#/ipd)
- “Global Agro Product Directory” is an Agrochemical product directory, wherein all approved agrochemical products can be tracked. (https://www.chemrobotics.com/pesticides-directory/)
- MRL database has the maximum residue limits (MRLs) allowed for most pesticides used on major fruit and vegetable export crops and commodities in different counties like the USA, Europe, Japan, Canada, China, India, Australia, Israel, Malaysia, and Taiwan. Exporters can use this database to find out the residue limits in global markets. (https://www.chemrobotics.com/agropat/#/mrldir)
- “Brazil-Ag-Pedia” is a Brazil Agrochemical product directory, wherein all approved agrochemical products in Brazil can be tracked with all relevant information (https://www.chemrobotics.com/agropat/#/brazil_ag_pedia)

