Summary :
Janssen Pharmaceutical, a division of Johnson & Johnson, won an arbitrary victory against royalty choices arising from the licencing and marketing of daratumumab, an anti-CD38 monoclonal antibody therapy with numerous FDA-approved applications (FDA). A three-person tribunal conducted the confidential arbitration earlier this week.

Janssen Wins Darzalex Royalties Arbitration Battle Against Genmab
In this arbitration, two topics were discussed.
- The first is connected to a Janssen-Genmab licencing arrangement. Daratumumab is a drug that Genmab holds the rights to develop, manufacture, and market. While Janssen must pay Genmab sales royalties on the product in every nation where it is sold until all of Genmab’s associated patents expire or are invalidated, this did not apply to the Janssen-owned patent. Within the next decade, these patents will expire.’
- The second issue that went to arbitration was how these royalties would be divided with a third firm, Halozyme Therapeutics, in exchange for Halozyme’s formulation of daratumumab that was administered subcutaneously. Also in Janssen’s advantage, the ruling will allow the business to continue to self-regulate the situation by splitting payments made to each company. Janssen deducts the difference between proper royalties paid to Halozyme and payments made to Genmab prior to and as a result of this arbitration.
About Daratumumab
Daratumumab, sold under the brand name Darzalex, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells
Daratumumab was originally created by Genmab, but it is now being developed jointly by Genmab and Janssen Biotech, a Johnson & Johnson company that bought the drug’s worldwide marketing rights from Genmab. Daratumumab was designated as a breakthrough therapy medication for multiple myeloma in 2013.
For multiple myeloma, diffuse large B cell lymphoma, follicular lymphoma, and mantle cell lymphoma, it was given orphan drug designation.
- Mechanism of action
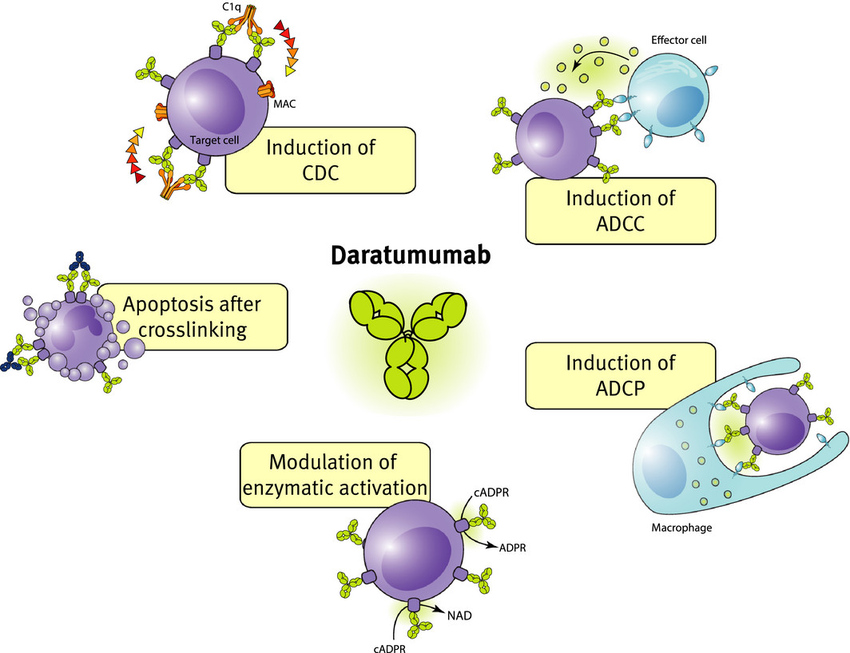
Mechanism of action
Daratumumab binds to CD38, causing cells to apoptose via antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, inhibition of mitochondrial transfer or antibody-dependent cellular phagocytosis.
Daratumumab is an antibody directed against a protein called CD38, which is found on the surface of multiple myeloma cells. Once daratumumab attaches itself to the cells expressing CD38, it summons the body’s immune system to attack and destroy those cells.
- Brand and Other Names :Darzalex
- Classes: Antineoplastics, Monoclonal Antibody; Antineoplastics, Anti-CD38 Monoclonal Antibodies
The antibody treatment was the first to be approved for the treatment of multiple myeloma and the sole approved treatment for light-chain amyloidosis. The medication works by attaching antibodies to CD38 molecules, which triggers an immune response and apoptosis in tumour cells.
The medicine has been granted the brand name Darzalex by Janssen, with the purpose of using it in conjunction with other drugs and personalising treatment to the indication. Darzalex Faspro is a medication that is sold in combination with hyaluronidase-fihj.
To accurately discuss the outcomes of participants, the study protocol included follow-ups, the topic of Janssen’s update. These follow-ups, which were conducted at 38.6 months on average, contributed to the overall positive results. Progression-free survival in both arms was one of the key indicators of the drug’s success, along with a lack of safety concerns. The minimal residual disease negativity rate continued to stay high in comparison to patients that were dosed with lenalidomide and dexamethasone alone, who served as the control group.
About Genmab
Genmab A/S is a Danish biotechnology company, founded in February 1999 by Florian Schönharting, at the time managing director of BankInvest Biomedical venture fund.
Genmab is an international biotechnology company with a core purpose to improve the lives of people with cancer. For more than 20 years, Genmab’s vision to transform cancer treatment has driven its passionate, innovative and collaborative teams to invent next-generation antibody technology platforms and leverage translational research and data sciences, fueling multiple differentiated cancer treatments that make an impact on people’s lives. To develop and deliver novel therapies to patients, Genmab has formed 20+ strategic partnerships with biotechnology and pharmaceutical companies.
Genmab’s proprietary pipeline includes bispecific T-cell engagers, next-generation immune checkpoint modulators, effector function enhanced antibodies and antibody-drug conjugates.
About Janssen Pharmaceuticals
Janssen Pharmaceuticals is a pharmaceutical company headquartered in Beerse, Belgium, and wholly-owned by Johnson & Johnson. It was founded in 1953 by Paul Janssen.
In 1961, Janssen Pharmaceuticals was purchased by New Jersey-based American corporation Johnson & Johnson, and became part of Johnson & Johnson Pharmaceutical Research and Development (J&J PRD), now renamed to Janssen Research and Development (JRD), which conducts research and development activities related to a wide range of human medical disorders, including mental illness, neurological disorders, anaesthesia and analgesia, gastrointestinal disorders, fungal infection, HIV/AIDS, allergies and cancer. Janssen and Ortho-McNeil Pharmaceutical have been placed in the Ortho-McNeil-Janssen group within Johnson & Johnson Company
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)

