Summay :
EAMS scientific opinion issued to Global Blood Therapeutics (UK) Ltd for Voxelotor in the treatment of hemolytic anemia in patients with sickle cell disease.
MHRA awards a positive scientific opinion for voxelotor–a once-daily tablet for the treatment of haemolytic anaemia
Global Blood Therapeutics (GBT) has announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has awarded a positive scientific opinion–under the Early Access to Medicines Scheme (EAMS)–for voxelotor.
The treatment is an oral once-daily tablet under review by the MHRA for the treatment of haemolytic anaemia due to sickle cell disease (SCD) in adults and paediatric patients 12 years-of-age and older. Voxelotor will be offered as monotherapy or in combination with hydroxycarbamide.

Global Blood Therapeutics (GBT) has announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has awarded a positive scientific opinion–under the Early Access to Medicines Scheme (EAMS)–for voxelotor.
The positive opinion means that patients living with SCD–and meeting the eligibility criteria–can gain early-pre-license access to voxelotor, while the MHRA finalises its review of the Marketing Authorisation Application (MAA).
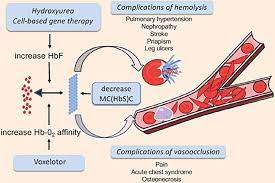
Voxelotor works by increasing haemoglobin’s affinity for oxygen. As oxygenated sickle haemoglobin does not polymerise, while voxelotor inhibits sickle haemoglobin polymerisation and the resultant destruction of red blood cells. GBT believes that voxelotor has the potential to modify the course of SCD. The treatment is approved in the US and has been given the seal of approval through a Priority Medicines (PRIME) designation from the European Medicines Agency.
SCD is a rare inherited blood disorder, affecting approximately 15,000 people in the UK. Patients experience progressive, serious complications and comorbidities, including end-organ damage, which can lead to a decreased quality of life and early death. There is no known cure for SCD, but there are treatments to help manage the condition.
“This decision marks a significant milestone for the sickle cell community in the UK,” said Arvind Agrawal, UK medical director at GBT. “The EAMS positive scientific opinion is a key step forward to meeting our goal of providing patients in the UK with the first oral treatment option that inhibits red blood cell sickling and has the potential to reduce acute and chronic complications of sickle cell disease. GBT is delighted with the progress to help eligible patients have access to this innovation as soon as possible.”
Voxelotor
Voxelotor, sold under the brand name Oxbryta, is a medication used for the treatment of sickle cell disease. Developed by Global Blood Therapeutics, voxelotor is the first hemoglobin oxygen-affinity modulator.
Dosage Forms & Strengths
tablet
- 500 mg
tablet for oral suspension
- 300 mg
Sickle Cell Disease
Indicated for treatment of sickle cell disease
1500 mg PO qDay
May take with or without hydroxyurea
Dosage Modifications
Renal impairment
- Mild, moderate, or severe: No dose adjustment required
- End-stage renal disease: Not studied
Hepatic impairment
- Mild-to-moderate (Child-Pugh A or B): No dose adjustment required
- Severe (Child-Pugh C): Decrease to 1000 mg (tablets) PO qDay or 900 mg (tablets for oral suspension) PO qDay
CYP3A4 inducers
- Avoid coadministration of strong or moderate CYP3A4 inducers
- If unable to avoid, adjust dose
- Strong CYP3A4 inducers: Increase to 2500 mg PO qDay
- Moderate CYP3A4 inducers: Increase to 2000 mg PO qDay
Contraindicated (1)
- lonafarnibvoxelotor will increase the level or effect of lonafarnib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated. Lonafarnib is a sensitive CYP3A4 substrate. Coadministration with strong or moderate CYP3A4 inhibitors is contraindicated.
Serious – Use Alternative (329)
- abametapir
- abemaciclib
- abiraterone
- acalabrutinib
- ado-trastuzumab emtansine
- alfentanil
- alfuzosin
- alpelisib
- alprazolam
- amiodarone
- amlodipine
- amobarbital
- apalutamide
- apixaban
- apremilast
- aprepitant
- aripiprazole
- armodafinil
- atazanavir
- atorvastatin
- avanafil
- avapritinib
- avatrombopag
- axitinib
- bedaquiline
- benzhydrocodone/acetaminophen
- benzphetamine
- bexarotene
- bortezomib
- bosentan
- bosutinib
- brentuximab vedotin
- brexpiprazole
- brigatinib
- bromocriptine
- budesonide
- buprenorphine
- buprenorphine buccal
- buprenorphine subdermal implant
- buprenorphine, long-acting injection
- buspirone
- busulfan
- butabarbital
- butalbital
- cabozantinib
- calcifediol
- calcitriol
- carbamazepine
- cariprazine
- ceritinib
- chlordiazepoxide
- chloroquine
- chlorpheniramine
- cilostazol
- citalopram
- clarithromycin
- clobazam
- clonazepam
- clorazepate
- cobicistat
- cobimetinib
- cocaine
- colchicine
- conivaptan
- copanlisib
- crizotinib
- cyclophosphamide
- cyclosporine
- dabrafenib
- dantrolene
- dapsone
- darifenacin
- darolutamide
- darunavir
- dasatinib
- deflazacort
- dexamethasone
- dexlansoprazole
- diazepam
- dihydroergotamine
- dihydroergotamine intranasal
- diltiazem
- disopyramide
- docetaxel
- doravirine
- doxorubicin
- doxorubicin liposomal
- dronabinol
- dronedarone
- duvelisib
- efavirenz
- elagolix
- elbasvir/grazoprevir
- eletriptan
- eliglustat
- elvitegravir
- elvitegravir/cobicistat/emtricitabine/tenofovir DF
- encorafenib
- entrectinib
- enzalutamide
- eplerenone
- ergoloid mesylates
- ergotamine
- erlotinib
- erythromycin base
- erythromycin ethylsuccinate
- erythromycin lactobionate
- erythromycin stearate
- escitalopram
- eslicarbazepine acetate
- esomeprazole
- estradiol
- estrogens esterified
- estropipate
- eszopiclone
- ethinylestradiol
- ethosuximide
- etoposide
- etravirine
- everolimus
- exemestane
- fedratinib
- felbamate
- felodipine
- fenofibrate
- fenofibrate micronized
- fenofibric acid
- fentanyl
- fentanyl intranasal
- fentanyl transdermal
- fentanyl transmucosal
- flibanserin
- flurazepam
- flutamide
- fosamprenavir
- fosphenytoin
- fostamatinib
- gefitinib
- glasdegib
- glecaprevir/pibrentasvir
- guanfacine
- haloperidol
- hydrocodone
- hydroxyprogesterone caproate
- ibrutinib
- idelalisib
- ifosfamide
- imatinib
- indinavir
- infigratinib
- irinotecan
- irinotecan liposomal
- isosorbide dinitrate
- isosorbide mononitrate
- isradipine
- istradefylline
- itraconazole
- ivabradine
- ivacaftor
- ivosidenib
- ixabepilone
- ixazomib
- ketamine
- lacosamide
- lansoprazole
- lapatinib
- larotrectinib
- lemborexant
- levoketoconazole
- levomilnacipran
- levonorgestrel oral
- linagliptin
- lomitapide
- lopinavir
- lorlatinib
- losartan
- lovastatin
- lumacaftor/ivacaftor
- lumefantrine
- lurasidone
- lurbinectedin
- macimorelin
- macitentan
- maraviroc
- medroxyprogesterone
- mefloquine
- mestranol
- metaxalone
- methadone
- methylergonovine
- methylprednisolone
- midazolam
- midostaurin
- mifepristone
- mirtazapine
- mitomycin
- mobocertinib
- modafinil
- montelukast
- nafcillin
- naldemedine
- naloxegol
- nefazodone
- nelfinavir
- netupitant/palonosetron
- nevirapine
- nicardipine
- nifedipine
- nilotinib
- nimodipine
- nisoldipine
- norethindrone
- norethindrone acetate
- olaparib
- ombitasvir/paritaprevir/ritonavir & dasabuvir (DSC)
- omeprazole
- ondansetron
- osimertinib
- ospemifene
- oxycodone
- paclitaxel
- paclitaxel protein bound
- palbociclib
- panobinostat
- paricalcitol
- pazopanib
- pemigatinib
- pentobarbital
- perampanel
- pexidartinib
- phenobarbital
- phenytoin
- pimavanserin
- pimozide
- pitolisant
- polatuzumab vedotin
- pomalidomide
- ponatinib
- praziquantel
- primaquine
- primidone
- progesterone micronized
- quazepam
- quetiapine
- quinidine
- quinine
- rabeprazole
- ranolazine
- regorafenib
- repaglinide
- ribociclib
- rifabutin
- rifampin
- rifapentine
- riociguat
- ritonavir
- rivaroxaban
- roflumilast
- rolapitant
- romidepsin
- ropivacaine
- ruxolitinib
- ruxolitinib topical
- salmeterol
- saquinavir
- saxagliptin
- secobarbital
- selumetinib
- sildenafil
- simvastatin
- siponimod
- sirolimus
- sofosbuvir/velpatasvir
- solifenacin
- sonidegib
- sorafenib
- St John’s Wort
- stiripentol
- sufentanil
- sufentanil SL
- sunitinib
- suvorexant
- tacrolimus
- tadalafil
- tamsulosin
- tasimelteon
- tazemetostat
- telotristat ethyl
- temsirolimus
- teniposide
- tetracycline
- tezacaftor
- theophylline
- tiagabine
- ticagrelor
- ticlopidine
- tinidazole
- tipranavir
- tofacitinib
- tolterodine
- tolvaptan
- toremifene
- trabectedin
- tramadol
- trazodone
- triazolam
- trimethoprim
- trimipramine
- ulipristal
- upadacitinib
- valbenazine
- vandetanib
- vardenafil
- velpatasvir
- vemurafenib
- venetoclax
- venlafaxine
- verapamil
- vilazodone
- vinblastine
- vincristine
- vincristine liposomal
- vinorelbine
- vorapaxar
- voriconazole
- vortioxetine
- voxilaprevir
- zolpidem
- zonisamide
Monitor Closely (9)
- atogepant
- daridorexant
- diazepam intranasal
- finerenone
- isavuconazonium sulfate
- lefamulin
- levamlodipine
- rimegepant
- voclosporin
Adverse Effects
>10%
Adults and adolescents ≥12 yr
- Headache (32%)
- Diarrhea (23%)
- Abdominal pain (23%)
- Nausea (19%)
- Rash (15%)
- Pyrexia (15%)
Pediatrics 4-11 yr
- Pyrexia (36%)
- Vomiting (33%)
- Rash (20%)
- Abdominal pain (18%)
- Diarrhea (18%)
- Headache (18%)
1-10%
Adults and adolescents ≥12 yr
- Drug hypersensitivity (<10%)
<1%
Adults and adolescents ≥12 yr
- Serious hypersensitivity

