APPLICATIONS OF CONTINUOUS FLOW PROCESS FOR BLOCKBUSTER INSECTICIDE MOLECULE (RYNAXYPYR) BY FMC CORPORATION

FMC
Aiming to develop a cost-effective process of making agrochemicals, nowadays, big agrochemical companies are developing continuous flow processes for blockbuster agrochemicals such as Rynaxypyr also known as Chlorantraniliprole.
Recent trends have been increasing to use continuous flow process applications in the agrochemical industry as well.
Recently, on June 11, 2020, FMC Corporation got a new International PCT patent application protecting (WO2020117493A1) continuous process for the preparation of Chlorantraniliprole (Rynaxypyr).
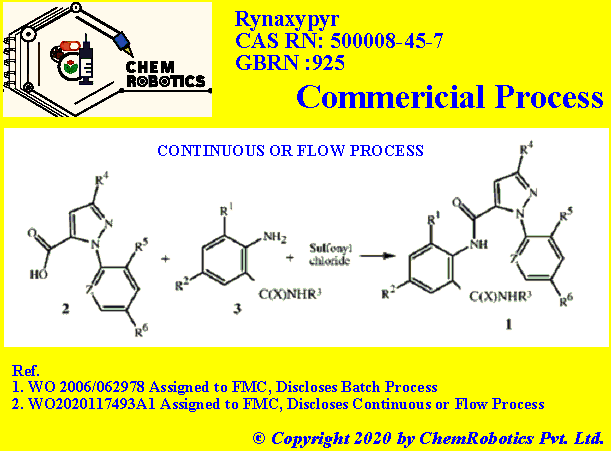
The disclosed method involves the combining compounds of Formulae 2 and 3 and a sulfonyl chloride in a continuous process. Please refer below process section for details.
FMC Agro Singapore PTE LTD. filed International Patent Application on Rynaxypyr (Chlorantraniliprole)
PCT Patent Application: WO2020117493
Publication Date: 11.06.2020
International Application No.: PCT/US2019/062778
Title: Method for preparing V-phenylpyrazole-1-carboxamides or V-pyridinylpyrazole-l -carboxamides by coupling carboxylic acids with anthranilamides in a continuous process.
International search authority cited one prior art WO2006/062978, which is assigned FMC, discloses a very similar process for the preparation of Rynaxypyr.
IP Scenario:
E.I. Du Pont protected the compound Chlorantraniliprole in two PCT patent applications, e.g. WO2003015518 & WO2003015519. Both PCT patent applications share the common priority patent applications. The Chlorantraniliprole is going to off-patent in many jurisdictions.
PCT Patent Publication WO 2003/015518 discloses the utility of V-acyl anthranilic acid derivatives of Formula I as arthropodicides.
FMC Rynaxypyr Process Details:
June 11, 2020, published PCT patent application claims (WO2020117493A1) claims the new continuous process for the preparation of Chlorantraniliprole. A method is disclosed for preparing compounds of Formula 1 by combining compounds of Formulae 2 and 3 and a sulfonyl chloride in a continuous process.
The said process comprises the step of
(1) combining formula (1) a carboxylic acid compound of Formula 2,
Rynaxypyr
(2) an aniline compound of Formula 3, wherein X, R1, R2, and R3 are as defined for the compound of Formula 1; and
(3) a sulfonyl chloride to form the compound of Formula 1; wherein the method includes a continuous process.
The patent also describes the advantage of a continuous process to produce N-phenylpyrazole-1-carboxamides compounds (Chlorantraniliprole) provides multiple advantages over the batch process that is practiced in WO 2006/062978 (prior art process).
Running continuously allows for faster throughput for a given reactor and helps improve the safety of the process by minimizing the amounts of reactive chemicals that could lead to a runaway reaction. While not all processes can be run in a continuous mode, due to impurity formation or handling issues, for example, it was discovered that the process described herein can be run in a continuous process in high yields and without the formation of new impurities, which is significant to meet the current global pesticide registration needs.
In addition, it was discovered that running in a continuous mode, particularly when feeding into a reaction zone of partially converted material, can be advantageous for the final product particle size, which improves its ease of filtration and product concentration. In a typical batch process, it is desirable to run with high concentrations of starting material, which often leads to the production of undissolved starting material solids that will impact the final product crystallization, resulting in small, hard to filter particles of product. By running continuously into a reaction zone of partially converted material, these solid starting materials quickly dissolve upon entering the reaction zone, essentially eliminating their impact on the crystallization. It was also discovered that the final product has a higher solubility in the reaction media as the level of conversion to the compound of Formula 1 increases, which also benefits crystallization and particle size by reducing the amount of supersaturation during crystallization.
About Manufacturing Process (Continuous OR Flow Process):
The pharmaceutical industry is highly dependent on the continuous flow process by using different types of reactors. It is considered the most accurate and beneficial technique for drug discovery. People have started the consumption of beverages and processed which undergoes this process to test the level of sucrose. Thus, the demand for flow chemistry market is growing.
Continuous production is a flow production method used to manufacture, produce, or process materials without interruption. Continuous production is called a continuous process or a continuous flow process because the materials, either dry bulk or fluids that are being processed are continuously in motion, undergoing chemical reactions or subject to mechanical or heat treatment. Continuous processing is contrasted with batch production.
The aim of the continuous manufacturing flow is to produce a flow production to manufacture, produce, or process materials uninterrupted.
The reason it is called a continuous process is that the materials, which can also be fluids, are being perpetually processed. During the process, materials will experience chemical reactions or mechanical or heat treatment.
The difference between continuous manufacturing and the process manufacturing (batch) is that instead of different stages of production being handled in a different area or even by another company, the entire process goes through one movement in one location, whilst being monitored for ongoing evaluation to improve the process.
The material is added at point A and leaves as a finished product further down the line at point B. Although, there might be offshoots as the same material can produce different products. The material may enter at point A, with some leaving at point B and the rest leaving at point C, to make two finished products.
In June 2018, Zhengzhou Well-known Instrument & Equipment Co. Ltd launched a glass reactor which will help in synthesizing new materials. It has excellent material and chemical properties. It will help in making the operation very stable and there will be no spark.
In January 2019, Parr Instrument Company has expanded its line of stirrers by adding a new robust spiral stirrer which can be customized for reactors with flat or tapered base geometries.
Advantages
- Cheaper labor rates since production will rely mostly on machinery than any other resource
- High accuracy from using machinery
- Easy and simple to organize this type of process
- Minimize your wastage
- Easier to manage inventory
- A high return on investments
Source: https://patentscope.wipo.int/search/en/search.jsf
For More details: Subscribe AgroPat* (http://chemrobotics.com/)
-
AgroPat provides techno-legal solutions to Agrochemical Discovery and Generic industry. (https://www.chemrobotics.com/agropat-lighter/login)
-
AgroPat platforms cover more than 4500 Active Ingredients (Al’s) with all information including synthesis (ROS), formulation, combination, innovator, patents including product patent, developer, utmost important biology data containing spectrum, MoA, DFU, toxicity, which can play a major role in decision making for any individual /company/ university/ industry.
-
- AgroPat Open Access – Free access to everyone,
- AgroPat Lite – With limited information as per business function needs,
- AgroPat ultimate – With all technical and business information,
-
-
Provide aboriginal information, which enables product developers to know about the state of the art in relevant subject matter, which includes innovator Patent / Non-Patent literature, which is critical for the generic industry.
-
Indian Pesticide Database (IPD) offers pesticide product registration/approval Information in various categories including Section 9(3), Section 9(3B), Section 9(4) and Bio-pesticides (https://www.chemrobotics.com/agropat/#/ipd)
-
“Global Agro Product Directory” is an Agrochemical product directory, wherein all approved agrochemical products can be tracked. (https://www.chemrobotics.com/pesticides-directory/)
-
MRL database has the maximum residue limits (MRLs) allowed for most pesticides used on major fruit and vegetable export crops and commodities in different counties like the USA, Europe, Japan, Canada, China, India, Australia, Israel, Malaysia, and Taiwan. Exporters can use this database to find out the residue limits in global markets. (https://www.chemrobotics.com/agropat/#/mrldir)
-
“Brazil-Ag-Pedia” is a Brazil Agrochemical product directory, wherein all approved agrochemical products in Brazil can be tracked with all relevant information (https://www.chemrobotics.com/agropat/#/brazil_ag_pedia)


