Synopsis :
-
Vaccine maker Bharat Biotech said that its nasal Covid-19 vaccine has been approved by the drug regulator for phase 3 clinical trials in the country.
-
An intra-nasal vaccine would not only be simple to administer but also reduce the use of needles and syringes. REUTERS/Stringer
The Drug Controller General of India on Friday gave approval to Bharat Biotech to conduct phase-3 trials for its Covid-19 intra-nasal vaccine (BBV154), sources said. The trials would evaluate the nasal vaccine for both the two-dose primary schedule and also to use as booster dose schedule, they said.
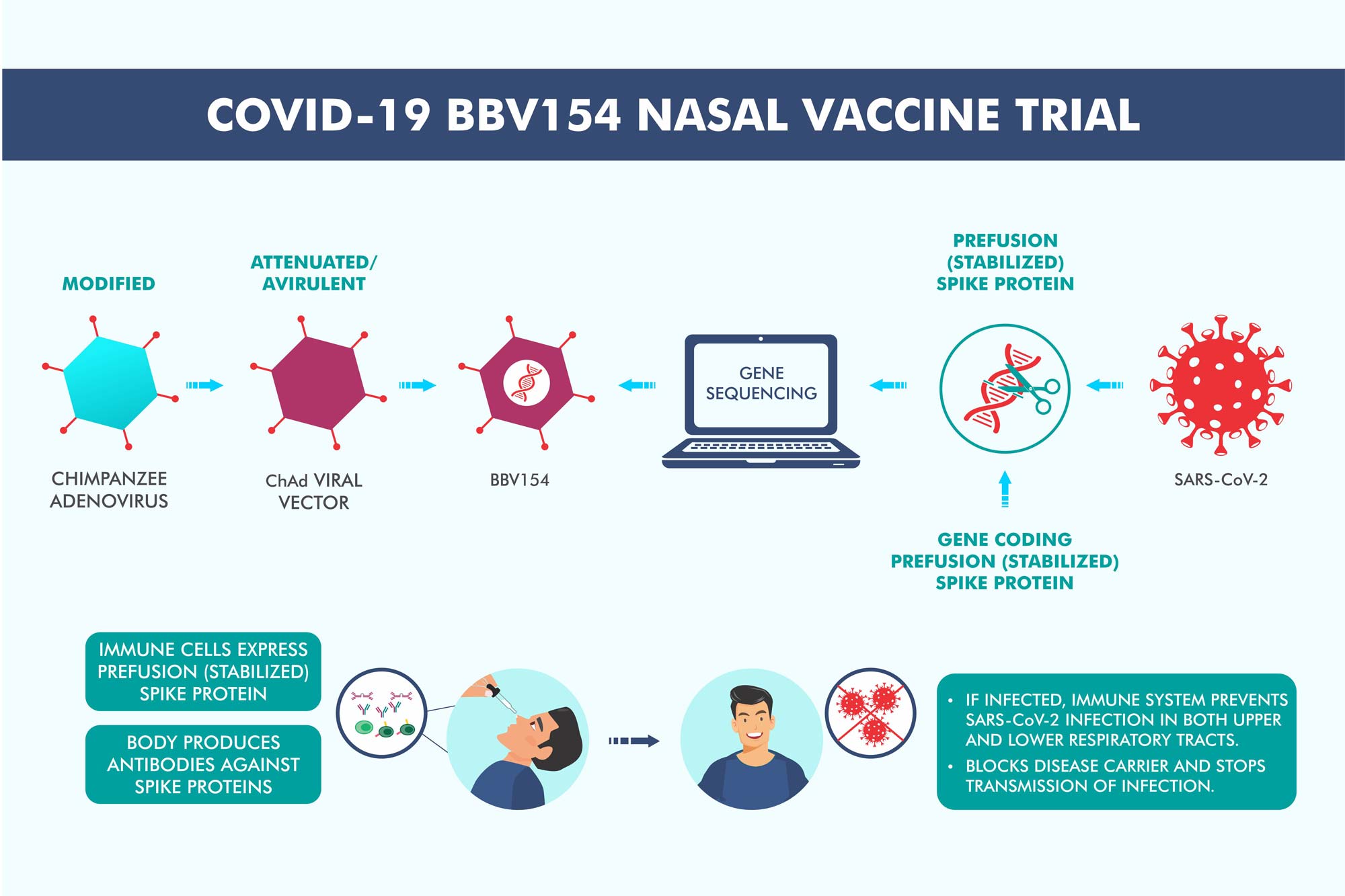
BBV154 (nasal covid vaccine) has received approval for phase-3 clinical trials. The trials will evaluate BBV154 nasal vaccine for both the two-dose primary schedule and booster dose schedule, the sources said. An intra-nasal vaccine would not only be simple to administer but also reduce the use of needles and syringes, among others. It would also impact the overall cost of a vaccination drive, chairman of Bharat Biotech Krishna Ella had said.

Bharat Biotech
The trials would evaluate the nasal vaccine for both the two-dose primary schedule and also to use as booster dose schedule, they said.
“BBV154 (nasal covid vaccine) has received approval for phase-3 clinical trials. The trials will evaluate BBV154 nasal vaccine for both the two-dose primary schedule and booster dose schedule,” the sources said.
An intra-nasal vaccine would not only be simple to administer but also reduce the use of needles and syringes, among others. It would also impact the overall cost of a vaccination drive, chairman of Bharat Biotech Krishna Ella had said.
BBV154
Key Attributes:
- An intranasal vaccine stimulates a broad immune response – neutralizing IgG, mucosal IgA, and T cell responses.
- Immune responses at the site of infection (in the nasal mucosa) – essential for blocking both infection and transmission of COVID-19.
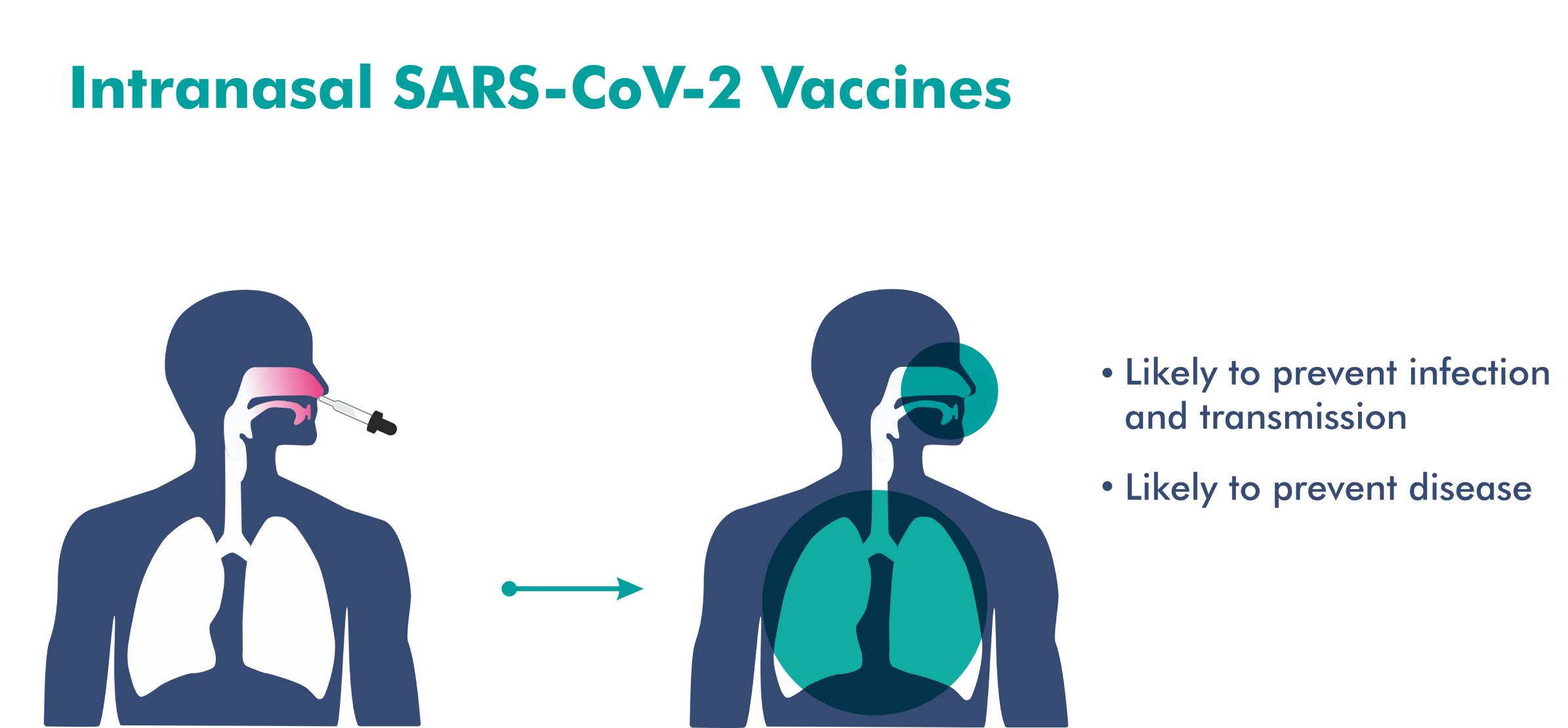
- The nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa.
- Non-invasive, Needle-free.
- Ease of administration – does not require trained health care workers.
- Elimination of needle-associated risks (injuries and infections).
- High compliance (Ideally suits for children’s and adults).
- Scalable manufacturing – able to meet global demand.