Summary –
BioRestorative Therapies, Inc. (the “Company” or “BioRestorative”) (NASDAQ:BRTX), a life sciences company focused on adult stem cell-based therapies, today announced it has entered into a Master Service Agreement with PRC Clinical, a contract research organization (CRO) specializing in clinical trial management to conduct BioRestorative’s Phase 2 clinical trial.

BioRestorative Therapies Announces CRO Agreement with PRC Clinical for its BRTX-100 Phase 2 Clinical Trial to Treat Chronic Lumbar Disc Disease
PRC Clinical is an all-inclusive CRO and has specialized expertise across regenerative medicine, CNS, ophthalmology, pulmonary and COVID-19, rare and orphan disease and more complex indications. Their innovative approach to executing studies for biotech and pharmaceutical companies combines high-touch human elements and cutting-edge technology with extensive experience and deep therapeutic knowledge. Pursuant to the agreement, PRC Clinical will manage BioRestorative’s Phase 2 clinical study of BRTX-100, the Company’s lead clinical product to treat chronic lumbar disc disease.
“This is a significant milestone in our mission to develop cell-based therapeutics and to become a clinical stage company. We believe that PRC’s renowned expertise in conducting clinicals trials and their use of innovative technology makes them an excellent CRO to partner with,” said Lance Alstodt, Chief Executive Officer of BioRestorative Therapies. “This is the most meaningful step the Company has taken to initiate our clinical trial. We should now expect a host of developments pertaining to the advancement of our clinical trial, ultimately leading to the read out of our primary endpoints.”
About BRTX-100
BRTX-100 is lead clinical candidate because:
- Prior human data provides insight into the potential efficacy of BRTX-100
- FDA authorized commencement of Phase 2 clinical trial
- Large indication with few comparable therapies
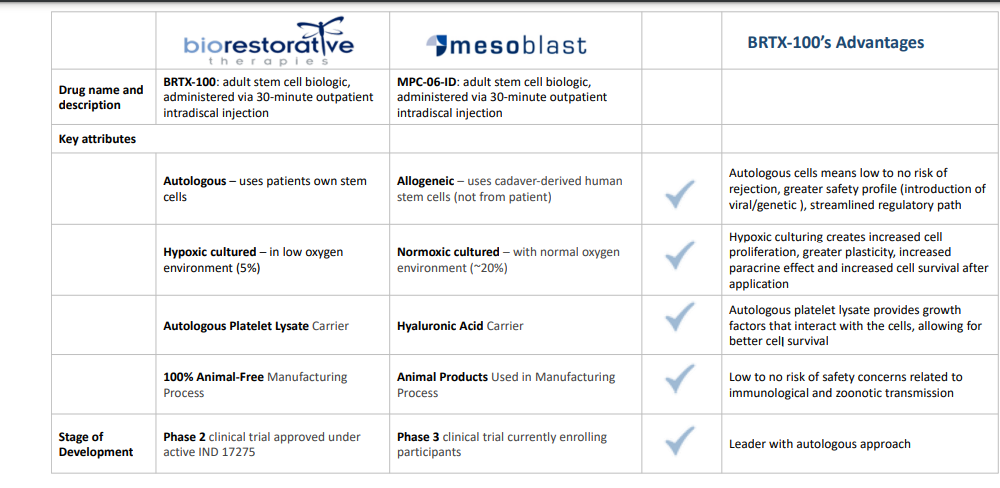
Comparison with Competitor
About BioRestorative Therapies, Inc.
BioRestorative Therapies, Inc. develops therapeutic products using cell and tissue protocols, primarily involving adult stem cells. Our two core programs, as described below, relate to the treatment of disc/spine disease and metabolic disorders:
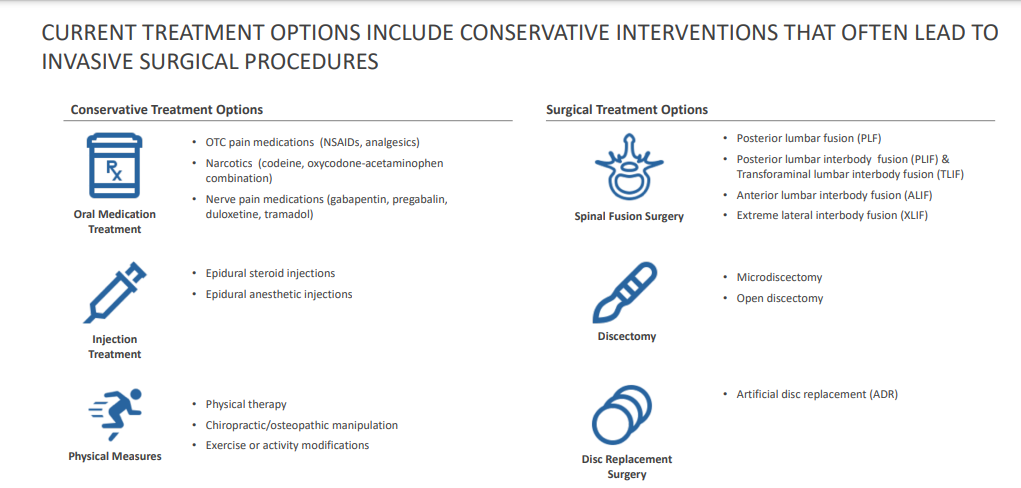
- Disc/Spine Program (brtxDISC™): Our lead cell therapy candidate, BRTX-100, is a product formulated from autologous (or a person’s own) cultured mesenchymal stem cells collected from the patient’s bone marrow. We intend that the product will be used for the non-surgical treatment of painful lumbosacral disc disorders or as a complementary therapeutic to a surgical procedure. The BRTX-100 production process utilizes proprietary technology and involves collecting a patient’s bone marrow, isolating and culturing stem cells from the bone marrow and cryopreserving the cells. In an outpatient procedure, BRTX-100 is to be injected by a physician into the patient’s damaged disc. The treatment is intended for patients whose pain has not been alleviated by non-invasive procedures and who potentially face the prospect of surgery. We have received authorization from the Food and Drug Administration to commence a Phase 2 clinical trial using BRTX-100 to treat chronic lower back pain arising from degenerative disc disease.
- Metabolic Program (ThermoStem®): We are developing a cell-based therapy candidate to target obesity and metabolic disorders using brown adipose (fat) derived stem cells to generate brown adipose tissue (“BAT”). BAT is intended to mimic naturally occurring brown adipose depots that regulate metabolic homeostasis in humans. Initial preclinical research indicates that increased amounts of brown fat in animals may be responsible for additional caloric burning as well as reduced glucose and lipid levels. Researchers have found that people with higher levels of brown fat may have a reduced risk for obesity and diabetes.

