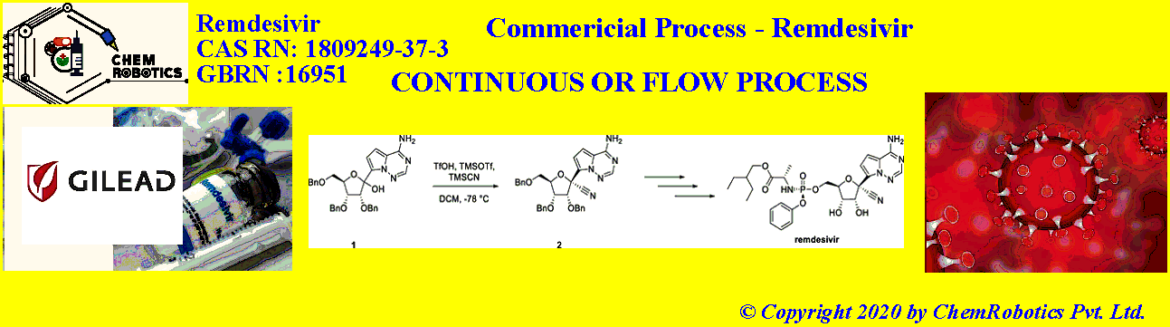
In the Recent Organic Process Research & Development (OPRD) publication on May 21, 2020 – Gilead Sciences published Continuous Flow Chemistry Application on Remdesivir (Covid-19 Drug).
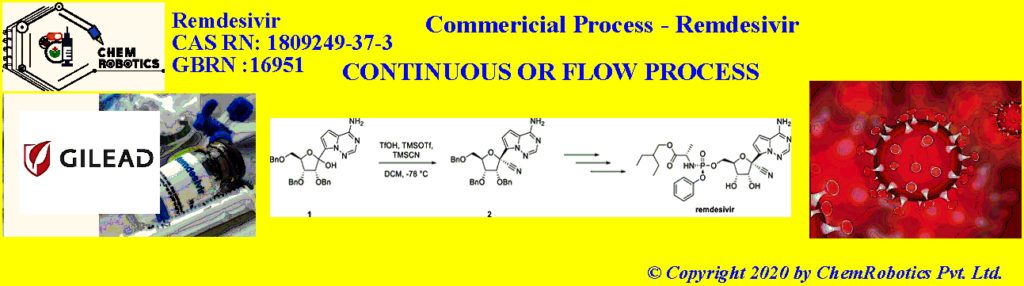
Continuous or Flow Process- Remdesivir
Said process new the involves an implementation of cyanation chemistry at manufacturing scales using batch equipment can be challenging due to the hazardous nature of the reagents employed, and the tight control of reaction parameters, including cryogenic temperatures, that help to afford acceptable selectivity and conversion for the desired reaction. Application of continuous flow chemistry offers a means to mitigate the risk associated with handling large amounts of hazardous reagents and to better control the reaction parameters.
Gilead Continuous-flow-chemistry-cyanation-remdesivir-COVID-19
Remdesivir is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. As of 2020, remdesivir is being tested as a specific treatment for COVID-19, and has been authorized for emergency use in the US, India, Singapore, and approved for use in Japan for people with severe symptoms. It also received approval in the UK in May 2020, however, it was going to be rationed due to limited supply. It may shorten the time it takes to recover from the infection.
Remdesivir has also demonstrated broad-spectrum antiviral activity against coronaviruses, including SARS-CoV and MERS-CoV in primary human cell culture models, and in vivo models of viral pathogenesis.
More recently, remdesivir has been found to possess in vitro inhibition of the novel 2019 coronavirus (SARS-CoV-2) responsible for the outbreak of COVID-19.
Two manufacturing processes for the preparation of compound 2 were developed: i) a batch cyanation process for pre-clinical and early-stage clinical Remdesivir demands; ii) a cyanation process using continuous flow for advanced phase clinical trials and commercial manufacturing.
Canada and colleagues have developed a batch synthesis process for the cyanation of a remdesivir precursor that can be operated at –30 °C. Previously, this reaction was performed using trifluoromethanesulfonic acid (TfOH), trimethylsilyl trifluoromethanesulfonate (TMSOTf), and trimethylsilyl cyanide (TMSCN). The team used trifluoroacetic acid (TFA) instead of TfOH and added a precooled mixture of TMSOTf and TMSCN in dichloromethane (DCM) to obtain good diastereoselectivity at –30 °C.
To allow for the production of larger amounts of the remdesivir precursor, the researchers developed a continuous flow process. In a small-scale lab system, they obtained a yield of 84 % at 99.8 % purity for 100 g of substrate. When the process was tested on a kilogram scale, the team found that the addition of TfOH was required to achieve the same results as in smaller-scale reactions. Using this additional catalyst, the process proved efficient for the large-scale synthesis of the cyanated remdesivir precursor. Only low volumes of dangerous reagents are present in the reactor at any given time, which reduces the risk of HCN exposure. The team obtained 68 kg of the desired product, which allowed the preparation of sufficient amounts of remdesivir for clinical evaluation.
PREVIOUSLY REPORTED PROCESS OF REMDESIVIR:
Reference: J. Med. Chem. 2017, 6, 1648–1661. Discovery and Synthesis of a Phosporamidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment off Ebola and Emerging Viruses.
The synthesis of remdesivir has been previously reported and proceeds through a 6-step sequence (Scheme 1) which includes the installation of a cyano group through the stereoselective addition of cyanide to the 1’-position of riboside 1. In the initial process, this transformation required cryogenic conditions (-78 °C) and had the potential to liberate significant quantities of hydrogen cyanide.
The cyanation reaction is performed through a combination of compound 1 (Scheme 1) in dichloromethane (DCM) with trifluoromethanesulfonic acid (TfOH), trimethylsilyl trifluormethanesulfonate (TMSOTf), and trimethylsilyl cyanide (TMSCN) while maintaining the reaction temperature at -78 °C. These reaction conditions afforded a clean reaction profile, with good diastereoselectivity (Table 1, entry 1).
The first challenge to be addressed was the requirement of cryogenic reaction temperatures. When the reaction was conducted at -15 °C, a rapid and significant decomposition of the starting material was observed along with reduced diastereoselectivity for the transformation (Table 1, entry 2). Alternative Brønsted acids were evaluated and trifluoroacetic acid (TFA) was found to be a promising lead (Table 1, entries 3–7), providing good solution purity of compound 2 and acceptable diastereoselectivity. Decreasing the reaction temperature to -30 °C was found to increase the solution purity as a result of decreased degradation of reactive intermediates while providing a modest improvement to the diastereoselectivity.
The order of addition was also found to impact the diastereoselectivity. For example, adding the TMSCN prior to the addition of TFA and TMSOTf significantly eroded the diastereoselectivity of the reaction (Table 1, entry 5 vs entry 9). Reagent equivalents were investigated and observed to play a role for reaction performance, as increasing the quantities of both TMSOTf and TMSCN to 6 equivalents increased the selectivity of the reaction, affording high diastereoselectivity of 93:7.
With the optimized conditions in hand, a larger scale experiment was performed. During the scale-up experiment, TMSOTf and TMSCN were each added over approximately 30 minutes to maintain the reaction at the target temperature of -30 °C. The 30 min addition time resulted in significant decomposition of the reaction mixture and lower diastereoselectivity.
This finding was addressed by first combining the starting material 1 in DCM with TFA at -30 °C, followed by adding a mixture of TMSOTf and TMSCN in DCM that had been pre-cooled to -30 °C. This new order of addition prevented the starting material from decomposing while maintaining acceptable diastereoselectivity. The stoichiometry of TMSOTf was revisited, and it was found that a decrease would result in compromised diastereoselectivity.
Having identified viable conditions for the formation of compound 2, attention was directed towards the isolation of the product. On the basis of a number of laboratory trials in solvent screening (over 40 single solvent or solvent combinations were investigated), it was determined that compound 2 exhibited favorable solid-state properties and could be selectively crystallized from either a mixed solvent system (such as ethyl acetate and heptane) or a single solvent (such as 2-propanol or toluene). Following additional development studies, including hazards assessments and safety studies, the batch cyanation process was successfully executed for the early deliveries of remdesivir (Table 2). This enabled the acceleration of the program at a time when there was an urgent need of remdesivir for clinical evaluation due to the escalating Ebola virus epidemic. For the planned large scale batch size, two key challenges needed to be addressed: improvement of the safety measures due to the increased operational hazards associated with the larger quantities of acidic reaction mixtures containing cyanide; and the increased operational time required to combine large quantities of compound 1/TFA with TMSOTf/TMSCN which could negatively impact diastereoselectivity and yield.
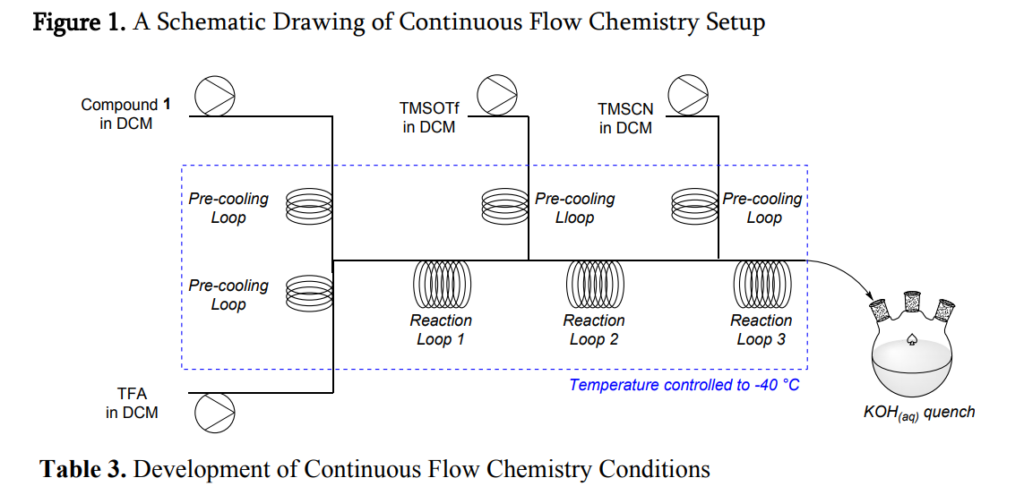
Feasibility Studies for the Preparation of Compound 2 via Continuous Flow Chemistry
Based on the above challenges associated with further increasing batch size, alternative processing modes were evaluated. Isolated literature reports discuss the use of continuous flow chemistry to perform C-glycosylations,9 O-glycosylations,10 and the use of cyanide in flow chemistry directly11, or by using a cyanide precursor.12 This mode of processing offers several unique advantages to traditional batch processing, including enabling large scale chemistry to be performed with reduced reaction volumes, and improved temperature control and mixing efficiency.13-17 Application of these advantages of continuous flow chemistry could, in principle, address the challenges associated with the batch cyanation process to prepare compound 2. For instance, smaller quantities of hazardous reaction mixture would be present during the reaction and could be rapidly quenched once the reaction stream exited the flow reactor, thereby mitigating a portion of the safety risks associated with the process. The continuous flow chemistry manufacturing mode would also enable processing facilities to perform the process at about -30 °C with relatively inexpensive cooling units. An appropriately designed flow reactor would improve mixing and cooling compared to batch mode chemistry, both of which could impact the process.
Information Source: Development of a Large-Scale Cyanation Process Using Continuous Flow Chemistry En Route to the Synthesis of Remdesivir. Authors: Tiago Vieira, †* Andrew C. Stevens, †* Andrei Chtchemelinine, ‡ Detian Gao, † Pavel Badalov, † and Lars Heumann ‡ †Gilead Alberta ULC, 1021 Hayter Road, Edmonton, Alberta T6S 1A1, Canada ‡Gilead Sciences, Inc. 333 Lakeside Drive, Foster City, California 94404, United States Publication Date: May 21, 2020 DOI: https://doi.org/10.1021/acs.oprd.0c00172
For More details: Subscribe AgroPat* (http://chemrobotics.com/)
ChemRobotics database can help the Agrochemical Discovery and generic industry in many ways. It is designed based on continuous industry feedback. It also helps the product development team to get information on the technical know-how of any agrochemical product.
-
AgroPat provides techno-legal solutions to Agrochemical Discovery and Generic industry. (https://www.chemrobotics.com/agropat-lighter/login)
-
AgroPat platforms cover more than 4500 Active Ingredients (Al’s) with all information including synthesis (ROS), formulation, combination, innovator, patents including product patent, developer, utmost important biology data containing spectrum, MoA, DFU, toxicity, which can play a major role in decision making for any individual /company/ university/ industry.
-
-
- AgroPat Open Access – Free access to everyone (with limited information)
- AgroPat Lite – Designed for business development function needs, user can access product overview
- AgroPat ultimate – With all technical and business information,
-
-
-
Provide aboriginal information, which enables product developers to know about the state of the art in relevant subject matter, which includes innovator Patent / Non-Patent literature, which is critical for the generic industry.
-
Indian Pesticide Database (IPD) offers pesticide product registration/approval Information in various categories including Section 9(3), Section 9(3B), Section 9(4) and Bio-pesticides (https://www.chemrobotics.com/agropat/#/ipd)
-
“Global Agro Product Directory” is an Agrochemical product directory, wherein all approved agrochemical products in 40 countries in a single platform can be accessed. – MRL database provides the maximum residue limits (MRLs) for various countries globally. (https://www.chemrobotics.com/pesticides-directory/)
-
MRL database has the maximum residue limits (MRLs) allowed for most pesticides used on major fruit and vegetable export crops and commodities in different counties like the USA, Europe, Japan, Canada, China, India, Australia, Israel, Malaysia, and Taiwan. Exporters can use this database to find out the residue limits in global markets. (https://www.chemrobotics.com/agropat/#/mrldir)
-
“Brazil-Ag-Pedia” is a Brazil Agrochemical product directory, wherein all approved agrochemical products in Brazil can be tracked with all relevant information (https://www.chemrobotics.com/agropat/#/brazil_ag_pedia)
- JARVIS Patent Database – A Competitor Patents Watch Database for Chemical and Agrochemical Segments.
- Technical supplier database (coming soon) -Technical, formulations details with their source of the supplier.
- Global Patent Watch – Open access platform, wherein all agrochemical and chemical patent updates can be accessed.
Product Brochure: CHEMROBOTICS PRODUCT BROCHURE Web Access link: https://www.chemrobotics.com/ User Manual of ChemRobotics: User Manual ChemRobotics - AgroPat Database – Sample Reports & Data
Communication & Interactive
Agrochemical Intelligence Database
ChemRobotics Pvt Ltd.
Email: support@chemrobotics.com, sales@chemrobotics.com, chemroboticstimes@chemrobotics.com