Corteva Agriscience new Nematicide Fluazaindolizine under the trade name Salibro Reklemel is set to be registered in Australia.
The APVMA (Australian Pesticides and Veterinary Medicines Authority) has evaluated the chemistry aspects of active constituent fluazaindolizine (physico-chemical properties, stability, identification, manufacturing process, quality control procedures, batch analysis results and analytical methods) and found them to be acceptable.
Fluazaindolizine is a novel nematicide, and the biochemical mode of action is currently unknown. It is selective for plant parasitic nematodes and is the first commercialised member of a new class of halogenated indole nematicides.
- Common name (ISO): Fluazaindolizine
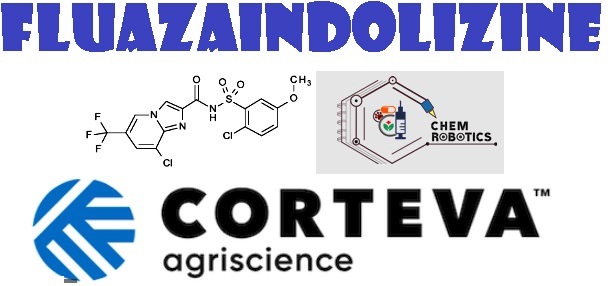
- IUPAC name: 8-chloro-N-[(2-chloro-5-methoxyphenyl)sulfonyl]-6-(trifluoromethyl)imidazo [1,2-a]pyridine-2-carboxamide
- CAS RN: 1254304-22-7
- Chemical Class: halogenated indole nematicides
- Salibro Reklemel active Nematicide will be available in 1 L to 200 L HDPE (high density polyethylene) containers.
- Formulation type: Suspension concentrate (SC)
- Active constituent concentration/s: 500 g/L fluazaindolizine
- Technical grade (96.4% purity) – solid, purified active (99.6% purity) – solid
- An acceptable daily intake (ADI) for fluazaindolizine was established at 0.4 mg/kg bw/d. This was based on an overall NOAEL of 36 mg/kg bw/d for mortality, reduced bodyweight gain, and histopathological findings (i.e. single cell necrosis and periportal vacuolation) from the liver of dogs fed diets of around 1,500 ppm in 3-and 12-month studies, or equivalent to a dose of 59 mg/kg bw/d.
- Acute Oral Neurotoxicity Study at 1.3 mg/kg bw based on a NOAEL of 125 mg/kg bw for inappetence and bodyweight loss.
Recommendations:
The APVMA Chemistry section has evaluated the chemistry of the active constituent fluazaindolizine and associated product Salibro Reklemel active Nematicide, including the physicochemical properties, specifications, manufacturing process, quality control procedures, packaging, stability, batch analysis results and analytical methods, and found them to be acceptable.
Based on a review of the chemistry and manufacturing details, the registration of Salibro Reklemel active Nematicide, and approval of the active constituent fluazaindolizine, are supported from a chemistry perspective.
Proposed claims and use pattern: Salibro Reklemel active Nematicide is a nematicide intended for control of root-knot nematodes in cucurbits, fruiting vegetables and root and tuber vegetables. Proposed application methods are by direct injection into drip/trickle irrigation or as in-furrow soil spray, incorporated by irrigation or mechanically, either pre- or post planting. Maximum application rates are 4 L/ha per year, either as a single treatment or as 2 applications of
2 L/ha.
Mode of action: Fluazaindolizine is a selective contact sulfonamide nematicide for the control of plant parasitic nematodes. It acts only on plant parasitic nematodes, and is not active against insect pests, plant pathogens or weeds. The mode of action is novel but remains unknown and has been designated as a Group N-UN: Unknown by the Insecticide Resistance Action Committee (IRAC 2018). Fluazaindolizine is efficacious broadly against pest nematodes species and specifically effective on root-knot nematodes (Meloidogyne spp.), reniform nematodes (Rotylenchulus spp.), dagger nematodes (Xiphinema spp.) and some lesion nematodes species (Pratylenchus spp.).
Overseas registrations: Registration of fluazaindolizine products are currently being sought in several other countries, including the USA, Canada, Japan and EU.
Toxicological assessment:
A full data package was assessed for fluazaindolizine. There are no objections on human health grounds to the approval of fluazaindolizine.
Toxicological assessment:
A full data package was assessed for fluazaindolizine. There are no objections on human health grounds to the approval of fluazaindolizine.
Evaluation of toxicology:
Chemical class:
Fluazaindolizine is a novel nematicide, and the biochemical mode of action is currently unknown. It is selective for plant parasitic nematodes, and is the first commercialised member of a new class of halogenated indole nematicides.
Pharmacokinetics:
Approximately half of the oral doses administered were absorbed, with the remainder excreted unchanged in faeces. Following absorption, fluazaindolizine was widely distributed. Levels in tissue were lower than levels in blood, and there was no potential for accumulation identified following repeat dosing. Fluazaindolizine was rapidly excreted in urine, largely as unchanged fluazaindolizine. There was limited metabolism, with major pathways involving demethylation, hydroxylation of the phenyl ring, and hydrolysis of the amide bond.
Acute toxicity (active constituent):
Fluazaindolizine has low acute toxicity after oral, dermal or inhalation administration. Fluazaindolizine was not irritating to the eyes and was not a skin sensitiser, however it was a moderate eye irritant in rabbits.
Acute toxicity (product):
Salibro Reklemel active Nematicide was of very low acute oral and inhalation toxicity, and low dermal toxicity. It was a slight eye and skin irritant, and not a skin sensitiser.
Repeat-dose toxicity:
In short- and long-term repeat oral dosing studies, the main adverse effects of fluazaindolizine were histopathological lesions mainly in the urinary tract among rodents (mice and rats), or in the liver in dogs.
The no observed adverse effect level in a 28 day dietary study in mice was 6000 ppm in the diet (equal to 1,105 mg/kg bw/day), the highest dose tested. In a 90 day study in mice, histopathological effects were seen in the liver, gall bladder and kidney at 3,000 ppm in the diet, and the no observed adverse effect level was 1k000 ppm in the diet, equivalent to 146 mg/kg bw/day.
In rats, histopathological effects on the kidney were seen after 90 days of feeding fluazaindolizine at concentrations above 3,000 ppm in the diet. The no observed effect level was 1,500 ppm, equal to 84 mg/kg bw/day.
Two studies were conducted in dogs, for 3 months and 12 months. At 4,000 ppm, liver changes included pigment accumulation, and gall bladder changes included pigmented luminar content.
Effects including mortality, reduced bodyweight gain and liver changes were observed in dogs fed 1,500 ppm, equivalent to 59 mg/kg bw/day. The overall no observed adverse effect level was 1,000 ppm, equivalent to 36 mg/kg bw/day.
Chronic toxicity and carcinogenicity:
Chronic dietary studies in mice and rats were conducted. In mice, deposition of amyloid was seen in several organs at 3,000 ppm (equivalent to 436 mg/kg bw/day). The overall no observed adverse effect level for the study was 1,000 ppm (equivalent to 142 mg/kg bw/day). Neoplasia was not recorded in this study, and the no observed adverse effect level for carcinogenicity was at the highest tested dose of 3,000 ppm (equivalent to 436 mg/kg bw/day).
The overall no observed adverse effect level in rats for dietary administration was 1,500 ppm (equivalent to 76 mg/kg bw/day), based on histopathological effects seen in the kidneys, along with urinalysis changes at 4,500 ppm (equivalent to 241 mg/kg bw/day). No evidence of neoplasia was recorded at any tested dose, and the no observed adverse effect level for carcinogenicity was the highest dose tested of 4,500 ppm (equivalent to 241 mg/kg bw/day).
Reproductive and developmental toxicity:
In a 2-generation reproductive toxicity study, the no observed adverse effect level for parental toxicity was 500 ppm in the diet (equivalent to 36 mg/kg bw/day), based on urinary tract effects. This was also the no observed adverse effect level for offspring toxicity, based on urinary tract effects. The no observed effect level for reproductive effects was 4,500 ppm (equivalent to 273 mg/kg bw/day), the highest dose tested.
Fluazaindolizine produced decreased foetal bodyweight and maternotoxic doses in rats, with a no observed adverse effect level for maternal toxicity, embyrotoxicity and foetotoxicity of 200 mg/kg bw/day. In rabbits, no embryo or foetotoxicity was observed, and the no observed adverse effect level was 120 mg/kg bw/day, the highest dose tested. The no observed adverse effect level for maternotoxicity was 30 mg/kg bw/day, based on the presence of reduced body weight gain at 120 mg/kg bw/day. Overall, it was concluded that fluazaindolizine is unlikely to be a teratogen.
Genotoxicity:
Fluazaindolizine was tested for genotoxicity in an adequate range of in vitro and in vivo assays. The overall weight of evidence indicates that fluazaindolizine is unlikely to be genotoxic in vivo.
Neurotoxicity/immunotoxicity:
Based on the results of acute and short-term studies in rats, it was concluded that fluazaindolizine is unlikely to be a neurotoxin.
The no observed adverse effect level for the humoral immune response was equal to 393 mg/kg bw/day in a 28 day rat study, significantly higher levels than other systemic toxicity effects.
Fluazaindolizine was also tested for its potential to interact with the endocrine system in a range of studies. All studies demonstrated that there was no interaction with estrogen or androgen-receptor pathways, or thyroid pathways.
Toxicity of metabolites and/or impurities:
The acute oral toxicity of several mouse, rat and plant metabolites was investigated, and found to be of low to very low acute oral toxicity. The weight of evidence suggested that all fluazaindolizine metabolites tested were unlikely to be genotoxic.
As metabolites were present in the urine of mice and rats at levels of less than 10% of the administered dose, specific toxicity could not be considered to have been adequately assessed using studies of the parent compound. As some residues were present in edible crops, repeat dose toxicity, reproduction and developmental studies were undertaken with certain metabolites. Overall they were considered likely to be equitoxic, and an ADI established for fluazaindolizine was considered to be protective for a dietary risk assessment.
- The OECD MRL calculator recommends an MRL of 0.15 mg/kg (STMR = 0.005 mg/kg, n = 69).
- An MRL rounded up to 0.2 mg/kg is recommended for fluazaindolizine on VC 0045 Fruiting vegetables, cucurbits in conjunction with a harvest withholding period of ‘Nil’, noting that the trials involved application up to harvest.
Table 1: Amendments to the APVMA MRL Standard:
| Fluazaindolizine | ||
| All other foods | 0.1 | |
| MO 0105 | Edible offal (mammalian) | *0.01 |
| PE 0112 | Eggs | *0.01 |
| VC 0045 | Fruiting vegetables, cucurbits | 0.2 |
| VO 0050 | Fruiting vegetables, other than cucurbits | 0.2 |
| HS 0783 | Galangal, rhizomes | 0.3 |
| MM 0095 | Meat (mammalian) | *0.01 |
| ML 0106 | Milks | *0.01 |
| PM 0110 | Poultry meat | *0.01 |
| PO 0111 | Poultry, edible offal of | *0.01 |
| VR 0075 | Root and tuber vegetables | 0.3 |
Source: Australian Pesticides and Veterinary Medicines Authority (APVMA): The Australian Pesticides and Veterinary Medicines Authority (APVMA) is the Australian Government statutory agency responsible for the management and regulation of all agricultural and veterinary chemical products in Australia.