Keypoints:
- Reddy’s Laboratories Ltd. has recently announced the launch of Carmustine for Injection, USP.
- Carmustine, sold under the brand name BiCNU among others, is a medication used mainly for chemotherapy.
- Carmustine is a nitrogen mustard β-chloro-nitrosourea compound used as an alkylating agent.
- The U.S. Food and Drug Administration approved carmustine — marketed under the trade name BiCNU — in March 1977.
- The cost for BiCNU intravenous powder for injection 100 mg is around $2,763 for a supply of 1 powder for injection.

Dr. Reddy’s Labs launches Carmustine for Injection Therapeutic Generic version BiCNU in US market.
Dr. Reddys Laboratories announced the launch of Carmustine for Injection, USP, a therapeutic equivalent generic version of BiCNU (carmustine for injection) approved by the U.S. Food and Drug Administration (USFDA).
The BiCNU brand and generic market had U.S. sales of approximately $19.4 million MAT for the most recent twelve months ending in August 2021 according to IQVIA Health.
Dr. Reddy’s Carmustine for Injection, USP is a lyophilized powder available as a package which includes a single-dose vial containing 100 mg Carmustine USP and a vial containing 3 mL sterile diluent.
What is Carmustine?
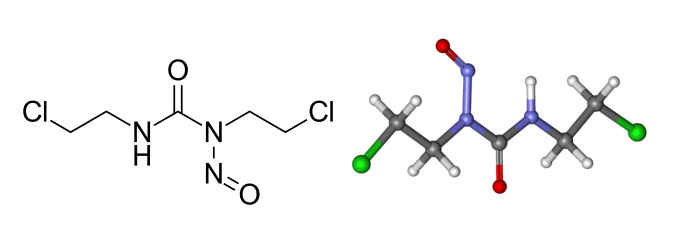
Structure of Carmustine.
IUPAC name
1, 3-Bis(2-chloroethyl)-1-nitrosourea
Other names
N,N’-Bis(2-chloroethyl)-N-nitrosourea, bis-chloroethylnitrosourea, BCNU
CAS Number
154-93-8
Chemical formula
C5H9Cl2N3O2
Molar mass
214.05 g·mol−1
Carmustine is the generic name for the trade name drug BiCNU®. In some cases, health care professionals may use the trade name BiCNU® when referring to the generic drug name carmustine.
Drug type: Carmustine is an anti-cancer (“antineoplastic” or “cytotoxic”) chemotherapy drug. This medication is classified as an “alkylating agent.”
Mechanism of action (MOA):
Carmustine causes cross-links in DNA and RNA, leading to the inhibition of DNA synthesis, RNA production and RNA translation (protein synthesis). Carmustine also binds to and modifies (carbamoylates) glutathione reductase. This leads to cell death.
What Carmustine Is Used For?
Used to treat certain types of brain tumors; glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma and metastatic brain tumors.
Other cancers treated with carmustine include multiple myeloma, Hodgkin’s disease, non-Hodgkin’s lymphomas, and may be used on the skin (topically) for cutaneous T-cell lymphoma.
Note: If a drug has been approved for one use, physicians sometimes elect to use this same drug for other problems if they believe it may be helpful.
How Carmustine Is Given?
Carmustine is usually given by an infusion into a vein (intravenous, IV).
There is no pill form of this medication. There is a form of this medication (Gliadel® wafer) that can be placed and left in the cavity after surgical removal of a brain tumor. The carmustine wafer allows for the delivery of the drug directly to the site of the brain tumour. (See separate listing “carmustine wafer” for more details regarding this formulation).
The amount of carmustine that you will receive depends on many factors, including your height and weight, your general health or other health problems, and the type of cancer or condition being treated. Your doctor will determine your dose and schedule.
Side Effects:
Important things to remember about the side effects of carmustine:
Most people do not experience all of the side effects listed. Side effects are often predictable in terms of their onset and duration. Side effects are almost always reversible and will go away after treatment is complete. There are many options to help minimize or prevent side effects.
There is no relationship between the presence or severity of side effects and the effectiveness of the medication. The side effects of carmustine and their severity depend on how much of the drug is given. In other words, high doses may produce more severe side effects.
The following side effects are common (occurring in greater than 30%) for patients taking carmustine:
Nausea and vomiting, usually within 2-4 hours of infusion, lasting for about 4-6 hours. Anti-nausea medication is given prior to infusion to prevent or decrease this side effect.
Facial flushing
Pain and burning at the injection site. (Can be relieved by diluting the drug, let your health care provider know if you are experiencing pain during the infusion).
Low blood counts. Your white blood cells and platelets may temporarily decrease. This can put you at increased risk for infection, and/or bleeding. This effect is usually delayed, onset 2 weeks after does with nadir 5-6 weeks later.
The following side effects are less common (occurring in 10-29%) for patients receiving carmustine:
Increases in blood tests measuring liver function, return to normal once treatment is stopped (see liver problems).
Low red blood cell count (anemia).
Low blood pressure (hypotension) with high dose therapy.
Dizziness, loss of coordination.
Eye problems: temporary redness and/or blurring, retinal bleeding.
Delayed effects:
Pulmonary toxicity (damage to the lungs) is uncommon in low doses of carmustine. However it is more common with cumulative or high doses. This toxicity may be delayed up to 3 years after treatment. A history of lung disease may increase the risk of this reaction, or use of other lung-toxic drugs. Your doctor will check your lung function prior to the start of carmustine and will order periodic checks (pulmonary function tests), particularly if you are receiving high doses of carmustine.
There is a slight risk of developing a blood cancer such as leukemia after taking carmustine. Talk to your doctor about this risk.Not all side effects are listed above, some that are rare (occurring in less than 10% of patients) are not listed here. However, you should always inform your health care provider if you experience any unusual symptoms
FDA approval to Carmustine.
The U.S. Food and Drug Administration approved carmustine — marketed under the trade name BiCNU — in March 1977. This chemotherapy drug damages the DNA of cancer cells, preventing them from dividing and triggering their death.
Why do we need its generic version?
As the cost for BiCNU intravenous powder for injection 100 mg is around $2,763 for a supply of 1 powder for injection, depending on the pharmacy you visit which is too expensive for the middle class or poor population.
The other drawback is, Prices are for cash paying customers only and are not valid with insurance plans.
Dr. Reddy`s Expertise.
Dr. Reddy’s API business thrives on the deep technical strengths established over the last 30+ years in the development and manufacture of complex APIs such as steroids, peptides, complex long chain molecules and highly potent APIs (HPAPIs / oncology drugs). This expertise is complemented by our prowess in intellectual property and regulatory affairs which helps us consistently meet and exceed regulatory standards. Dr. Reddy’s Abiraterone Acetate API is the outcome of the extensive expertise in R&D, IP, and Regulatory.
A key component in helping our customers be first to market is a responsive supply chain. We achieve this by making sure that all our facilities are operating efficiently and to the latest standards of quality, safety, and productivity. A strong interconnect between business and factories allows for a quick reaction to dynamic market changes, so that we can avert shortages and meet sudden surges in demand.
For more Information: Sign-in Websites for Agrochemical & Pharmaceutical Databases:
Website: https://www.chemrobotics.com/ (Agrochemical Databases)
Website: https://chemroboticspharma.com/ (Pharmaceutical Databases)

