Synopsis
Dr Reddy’s Valsartan Tablets are available in 40 mg in bottle count size of 30, and 80 mg, 160 mg and 320 mg tablets in bottle count sizes of 90
Homegrown pharma major Dr Reddy’s Laboratories on Thursday said it has launched Valsartan tablets, used for treatment of high blood pressure and heart failure, in the US market.
The Valsartan tablet is the generic therapeutic equivalent of Diovan, approved by the US Food and Drug Administration (USFDA), the company said in a regulatory filing.

Dr. Reddy To sale Valsartan in US Market
Dr Reddy’s Valsartan Tablets are available in 40 mg in bottle count size of 30, and 80 mg, 160 mg and 320 mg tablets in bottle count sizes of 90, it added.
Citing IQVIA Health data, the company said the Diovan brand and generic market had US sales of approximately USD 150 million for the most recent 12 months ended October 2021, it added.
About Valsartan
It is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versions are available as the combination valsartan/hydrochlorothiazide, valsartan/amlodipine, valsartan/amlodipine/hydrochlorothiazide, or valsartan/sacubitril.
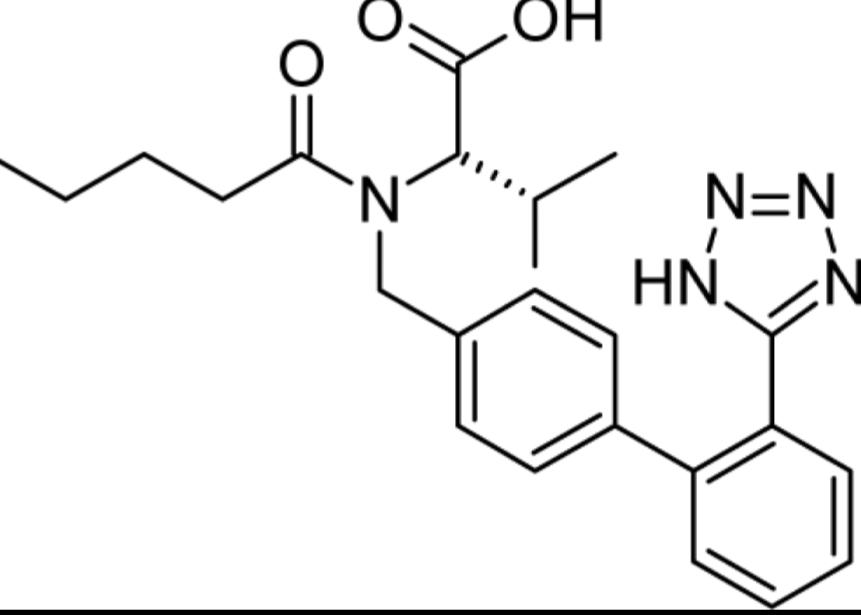
Structure of Valsartan
Medical uses
Valsartan is used to treat high blood pressure, heart failure, and to reduce death for people with left ventricular dysfunction after having a heart attack.
High blood pressure
It is a reasonable initial treatment for high blood pressure as are ACE inhibitors, calcium-channel blockers, and thiazide diuretics.
Heart failure
There is contradictory evidence with regard to treating people with heart failure with a combination of an angiotensin receptor blocker like valsartan and an angiotensin-converting enzyme inhibitor, with two major clinical trials showing a reduction in death, and two others showing no benefits, and more adverse effects including heart attacks, hypotension, and renal dysfunction.
Diabetic kidney disease
In people with type 2 diabetes and high blood pressure or albumin in the urine, Valsartan is used to slow the worsening and the development of end-stage kidney disease.
Mechanism of Action
Valsartan blocks the actions of angiotensin II, which include constricting blood vessels and activating aldosterone, to reduce blood pressure. The drug binds to angiotensin type I receptors (AT1), working as an antagonist. This mechanism of action is different than that of the ACE inhibitor drugs, which block the conversion of angiotensin I to angiotensin II. As valsartan acts at the receptor, it can provide more complete angiotensin II antagonism since angiotensin II is generated by other enzymes as well as ACE. Also, valsartan does not affect the metabolism of bradykinin like ACE inhibitors do.
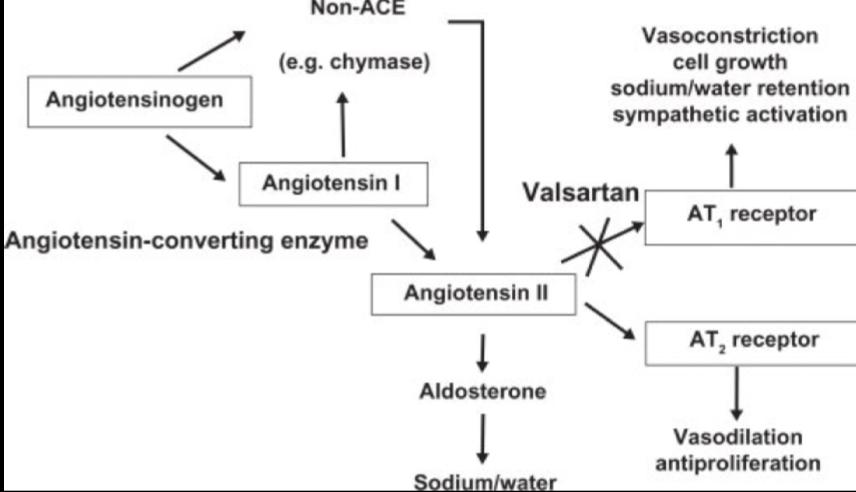
Mechanism of Action of Valsartan
Interactions
The U.S. prescribing information lists the following drug interactions for valsartan:
- Other inhibitors of the renin-angiotensin system may increase the risks of low blood pressure, kidney problems, and hyperkalemia.
- Potassium sparing diuretics, potassium supplements, salt substitutes containing potassium may increase the risk of hyperkalemia.
- NSAIDs may increase the risk of kidney problems and may interfere with blood pressure-lowering effects.
- Valsartan may increase the concentration of lithium.
- Valsartan and other angiotensin-related blood pressure medications may interact with the antibiotics co-trimoxazole or ciprofloxacin to increase risk of sudden death due to cardiac arrest.
Food interaction
With the tablet, food decreases the valsartan tablet taker’s exposure to valsartan by about 40% and peak plasma concentration (Cmax) by about 50%, evidenced by AUC change
Contraindications
The packaging for valsartan includes a warning stating the drug should not be used with the renin inhibitor aliskiren in people with diabetes. It also states the drug should not be used in people with kidney disease
About Dr. Reddy
Dr. Reddy’s Laboratories is an Indian multinational pharmaceutical company located in Hyderabad, Telangana, India. The company was founded by Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy’s manufactures and markets a wide range of pharmaceuticals in India and overseas. The company has over 190 medications, 60 active pharmaceutical ingredients (APIs) for drug manufacture, diagnostic kits, critical care, and biotechnology products.
Dr. Reddy’s began as a supplier to Indian drug manufacturers, but it soon started exporting to other less-regulated markets that had the advantage of not having to spend time and money on a manufacturing plant that would gain approval from a drug licensing body such as the U.S. Food and Drug Administration (FDA). By the early 1990s, the expanded scale and profitability from these unregulated markets enabled the company to begin focusing on getting approval from drug regulators for their formulations and bulk drug manufacturing plants – in more-developed economies. This allowed their movement into regulated markets such as the US and Europe. In 2014, Dr. Reddy Laboratories was listed among 1200 of India’s most trusted brands according to the Brand Trust Report 2014, a study conducted by Trust Research Advisory, a brand analytics company.
By 2007, Dr. Reddy’s had seven FDA plants producing active pharmaceutical ingredients in India and seven FDA-inspected and ISO 9001 (quality) and ISO 14001 (environmental management) certified plants making patient-ready medications – five of them in India and two in the UK.
In 2010, the family-controlled Dr Reddy’s denied that it was in talks to sell its generics business in India to US pharmaceutical giant Pfizer, which had been suing the company for alleged patent infringement after Dr Reddy’s announced that it intended to produce a generic version of atorvastatin, marketed by Pfizer as Lipitor, an anti-cholesterol medication. Reddy’s was already linked to UK pharmaceuticals multinational Glaxo Smithkline.

