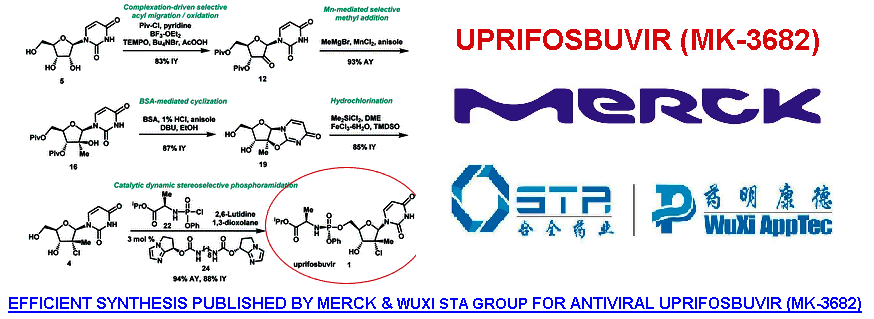
Department of Process Research and Development, Merck & Co., Inc., Rahway, New Jersey & WuXi STA, 90 Delin Road, Waigaoqiao Free Trade Zone, Shanghai 200131, China came up with an efficient route for the HCV antiviral agent Uprifosbuvir.
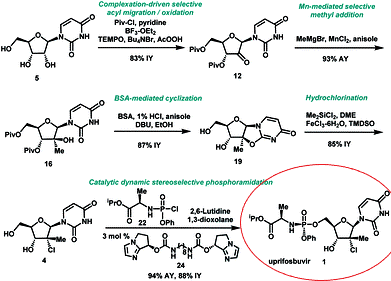
Uprifosbuvir new synthetic route was developed in 5 steps from readily available uridine in 50% overall yield. This concise synthesis was achieved by the development of several synthetic methods: (1) complexation-driven selective acyl migration/oxidation; (2) BSA-mediated cyclization to anhydrouridine; (3) hydrochlorination using FeCl3/TMDSO; (4) dynamic stereoselective phosphoramidation using a chiral nucleophilic catalyst. The new route improves the yield of Uprifosbuvir 50-fold over the previous manufacturing process and expands the toolset available for the synthesis of antiviral nucleotides.
UPRIFOSBUVIR_MERCK & WUXI STA
As highlighted by the COVID-19 pandemic, the availability of efficient antiviral treatments remains a starkly unmet medical need for most viral infections. Among existing antiviral drugs, several have been approved for the treatment of more than one viral disease and already approved antiviral drugs can sometimes be repurposed as treatments for emerging infectious diseases. This potential for emergency use underscores the need for a large and diverse stockpile of antiviral agents and the importance of efficient manufacturing processes to enable a rapid response to a potentially massive increase in demand.
Uprifosbuvir (MK-3682) is an antiviral drug developed for the treatment of Hepatitis C. It is a nucleotide analogue that acts as an NS5B RNA polymerase inhibitor. It is currently in Phase III human clinical trials.
In June 2014 – Merck & Co. obtained Uprifosbuvir from Idenix Pharmaceuticals with the $3.85 billion acquisition.

Idenix Pharmaceuticals
Merck’s development plans for Uprifosbuvir (MK3682)—a nucleotide (‘nuke’) NS5b polymerase inhibitor acquired along with the rest of Idenix for $3.9bn in 2014—is to combine it with two of its other HCV drugs in a cocktail that can be used in all patients, regardless of their virus genotype.
Uprifosbuvir has a similar structure to Gilead Sciences’ Sovaldi (sofosbuvir) and is in multiple trials alongside Merck’s NS3/4A protease inhibitor grazoprevir, one of the components in Merck’s two-drug Zepatier product, and experimental NS5a blocker ruzasvir (MK8408).
Uprifosbuvir is an NS5b inhibitor developed for the treatment of HCV, representing a class of 2′-branched nucleosides modified with a ProTide sidechain. Many other nucleoside antivirals also contain 2′, 3′, or 4′-modifications of the ribose core, presenting formidable synthetic challenges that have been approached through de novo synthesis. The alternative and more direct strategy to functionalize preexisting nucleosides is less developed.
In summary, we have developed a highly efficient route to HCV antiviral Uprifosbuvir 1 in five easily scalable steps from the readily available raw material uridine 5 in 50% overall yield, which represents a 50-fold yield improvement over the initial multi-kilo route. This achievement was made possible by the development of several synthetic methods: (1) complexation-driven selective acyl migration/oxidation; (2) BSA-mediated cyclization to anhydrouridine; (3) hydrochlorination using Me2SiCl2 and FeCl3/TMDSO; (4) dynamic stereoselective phosphoramidation using a chiral nucleophilic imidazole carbamate catalyst.
Synthesis_Uprifosbuvir
Scheme 1 Summary of Uprifosbuvir synthesis. AY = assay yield; IY = isolated yield.
About WuXi STA: WuXi STA, a subsidiary of WuXi AppTec company, is a leading pharmaceutical development and manufacturing capability and technology platform company serving the life science industry, with four sites in China and one site in the United States. As a premier Contract Development and Manufacturing Organization (CDMO), STA offers our worldwide partners efficient, flexible, and high-quality solutions for small molecule Active Pharmaceutical Ingredients (APIs) and finished dosage forms. Our globally integrated CMC platform supports small molecules, oligonucleotides, peptides, and antibody-drug conjugates from preclinical to commercial.
Source: https://pubs.rsc.org/en/content/articlelanding/2021/sc/d1sc01978c#!divAbstract

