
GSK manufactured First ever Malaria Vaccine gets nod from WHO for African Countries
· First ever novel vaccine for malaria approved by WHO for African Countries
· RTS,S/AS01 (trade name Mosquirix is a recombinant protein-based malaria vaccine.
· In October 2021, the vaccine was endorsed by the World Health Organization (WHO) for “broad use” in children, making it the first malaria vaccine candidate.
· Vaccine was conceived of and created in the late 1980s by scientists working at SmithKline Beecham Biologicals (now GlaxoSmithKline (GSK) Vaccines) laboratories in Belgium.
· Vaccine is developed GSK collaboration with Walter Reed Army Institute of Research in the U.S. state of Maryland.
· Vaccine Project: Funded in part by the PATH Malaria Vaccine Initiative and the Bill and Melinda Gates Foundation.
· Approved by the European Medicines Agency (EMA) in July 2015.
· WHO recommendation:On 23 October 2015, WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Committee (MPAC) jointly recommended a pilot implementation of the vaccine in Africa.
· This pilot project for vaccination was launched on 23 April 2019 in Malawi, on 30 April 2019 in Ghana, and on 13 September 2019 in Kenya
· Mosquirix is a vaccine that is given to children aged 6 weeks to 17 months to help protect against malaria caused by the parasite Plasmodium falciparum.
WHO has allowed widespread use of the world’s first malaria vaccine, which has reduced disease as part of a pilot project in parts of Africa. But its efficacy is modest, and the hunt for newer vaccines continues.
Malaria kills approximately 438,000 people a year worldwide and causes illness in hundreds of millions more, with most deaths occurring among children living in sub-Saharan Africa. Although existing interventions have helped to reduce malaria deaths significantly over the past 15 years, a well-tolerated and effective vaccine with an acceptable safety profile could add an important complementary tool for malaria control efforts. To date, no vaccine against malaria has been licensed for use.
RTS,S/ASO1 (RTS.S), trade name Mosquirix, which was endorsed by the World Health Organisation (WHO) on Wednesday (October 6), is the first and, to date only, vaccine shown to have the capability of significantly reducing malaria, and life-threatening severe malaria, in tests on young African children.
The vaccine acts against P. falciparum, the most deadly malaria parasite globally, and the most prevalent in Africa. Among children who received 4 doses in largescale clinical trials, the vaccine was able to prevent approximately 4 in 10 cases of malaria over a 4-year period.
This is the first malaria vaccine that has completed the clinical development process, and received a positive scientific opinion from the European Medicines Agency (EMA).
It is also the first malaria vaccine to be introduced by three national ministries of health through their childhood immunization programmes — more than 800,000 children in Ghana, Kenya, and Malawi have been vaccinated, and are benefiting from the added protection provided by the vaccine as part of a pilot programme.
Other recent clinical evidence shows that strategic delivery of the vaccine just prior to the high malaria transmission season in areas where malaria is highly seasonal can optimize impact and markedly reduce mortality, especially when combined with other recommended malaria control interventions.
The global burden of malaria
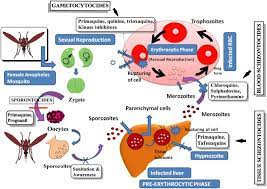
Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes. It is preventable and curable.
Still, in 2019, there were an estimated 229 million cases of malaria worldwide, and the estimated number of malaria deaths that year stood at 409,000.
Children aged under 5 years are the most vulnerable group affected by malaria; in 2019, they accounted for 67% (274,000) of all malaria deaths worldwide.
In 2019, India had an estimated 5.6 million cases of malaria compared to about 20 million cases in 2000, according to WHO.
How the vaccine can help
WHO’s recommendation is based on the advice of its two global advisory bodies, one for immunization and the other for malaria.
WHO has recommended that in the context of comprehensive malaria control, the RTS,S/AS01 malaria vaccine be used for the prevention of P. falciparum malaria in children living in regions with moderate to high transmission as defined by it.
The malaria vaccine should be provided in a schedule of 4 doses in children from 5 months of age for the reduction of malaria disease and burden.
The next steps for the WHO-recommended malaria vaccine will include funding decisions from the global health community for broader rollout in endemic countries, and country decision-making on whether to adopt the vaccine as part of national malaria control strategies.
A vaccine is a breakthrough addition to the malaria toolkit and can help get malaria control back on track.
Countries that have eliminated malaria
Globally, the elimination net is widening, with more countries moving towards the goal of zero malaria. In 2019, 27 countries reported fewer than 100 indigenous cases of the disease, up from 6 countries in 2000.
Countries that have achieved at least 3 consecutive years of zero indigenous cases of malaria are eligible to apply for the WHO certification of malaria elimination. Over the last two decades, 11 countries have been certified by the WHO Director-General as malaria-free: United Arab Emirates (2007), Morocco (2010), Turkmenistan (2010), Armenia (2011), Sri Lanka (2016), Kyrgyzstan (2016), Paraguay (2018), Uzbekistan (2018), Algeria (2019), Argentina (2019), and El Salvador (2021).
Why is a vaccine against malaria important?
Malaria is one of the deadliest diseases in human history, having claimed millions of lives. Even today, it kills over four lakh every year, according to WHO. This is still a huge improvement from 20 years ago, when close to twice this number were dying of the disease.
Malaria is most endemic in Africa, with Nigeria, Congo, Tanzania, Mozambique, Niger and Burkina Faso together accounting for over half the yearly deaths.
In the last few years, significant progress has been made in reducing its impact. A few countries have also been able to eliminate malaria, mainly through spray of insecticides to kill mosquitoes, and cleaning up areas where mosquitoes breed. In the last 20 years, 11 countries have been declared by WHO as malaria-free, after zero cases were recorded in these countries for three consecutive years. These include the United Arab Emirates, Morocco, Sri Lanka and Argentina. In 2019, 27 countries reported less than 100 cases. Two decades ago, only six countries had less than 100.
India is one of the countries badly affected by the disease. Although deaths due to malaria have come down sharply in the last few years — officially these are only in hundreds now —infections continue to be in millions.
What is the vaccine that has been cleared for widespread use?
RTS,S/AS01 is the result of a partnership between GlaxoSmithKline and the global non-profit PATH’s Malaria Vaccine Initiative, with grant funds from the Bill & Melinda Gates Foundation. It is a recombinant protein vaccine, which means it includes DNA from more than one source. It targets a protein called circumsporozoite in Plasmodium falciparum — the deadliest malaria parasite globally and the most prevalent one in Africa. It offers no protection against P vivax malaria, which predominates in many countries outside of Africa.
The vaccine is formulated with an adjuvant called AS01. It is designed to prevent the parasite from infecting the liver, where it can mature, multiply, and infect red blood cells, which can lead to disease symptoms.
The vaccine, which requires four injections, is for children under the age of five. Its efficacy is modest, as demonstrated in phase 3 trials from 2009 to 2014, on 15,000 young children and infants in 7 African countries. Four doses prevented 39% cases of malaria over 4 years of follow-up and 29% cases of severe malaria, with significant reductions also seen in overall hospital admissions.
RTS,S/AS01 (trade name Mosquirix) is a recombinant protein-based malaria vaccine
CAS RN: 149121-47-1
Innovator(Originator)- GSK {GlaxoSmithKline}
IP Scenario:
Patent Information: 1. Patent Number: WO9310152A1
Assignee: Smithkline Beecham Biologicals SA, Belgium
Publication Date: 1993-05-27
Est.Exp.: 2012-11-11
Application Number: WO1992-EP2591
Application Date: 1992-11-11
Equivalents: WO9310152A1; AU9229278A; EP614465A1; JP07501213T; EP614465B1; AT177755T; ES2129461T3; CA2123612C; JP3954643B2; ZA9208770A; US5928902A; AU9714717A; AU712409B2; US6169171B1; HK1012405A1; JP2007209343A; JP4241846B2
Indian Equivalents: Not Found
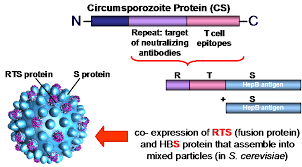
Title: Hybrid protein with Plasmodium CS protein sequence and hepatitis B surface antigen sequence, and use for vaccine against malaria
By: De Wilde, Michel; Cohen, Joseph
Abstract: A novel hybrid protein is provided which comprises a portion of the CS protein of P. falciparum and the surface antigen of Hepatitis B virus. The use of this protein for vaccination purposes is disclosed.
Protein Sequence
The protein sequence
Naming the drug
RTS,S is a scientific name given to this malaria vaccine candidate and represents its composition. The ‘R’ stands for the central repeat region of Plasmodium (P.) falciparum circumsporozoite protein (CSP); the ‘T’ for the T-cell epitopes of the CSP; and the ‘S’ for hepatitis B surface antigen (HBsAg).
Why has it taken so long to develop a vaccine against malaria?
Although there have been decades of research, and over 20 candidates have entered clinical trials in the last few years, the best prevention of malaria remains the use of mosquito nets — which do nothing to eradicate malaria. Mosquirix itself is the result of more than 30 years of research and development.
“The difficulty in developing effective malaria vaccines stems largely from the complexity of the malaria-causing parasites’ life cycle, which includes mosquitoes, human liver, and human blood stages, and subsequent antigenic variations of the parasite. These parasites are also able to hide inside human cells to avoid being recognised by the immune system, creating further challenges,” a group of Australian and Chinese researchers wrote in an open-access journal last year.
They cited another challenge: “The most common mouse models of malaria employ the rodent-specific parasite species P. berghei, P. yoelii, and P. chabaudi… While they are still employed to model various manifestations of human disease, the immune response patterns observed in these models are not fully transferable to humans.
RTS,S Development
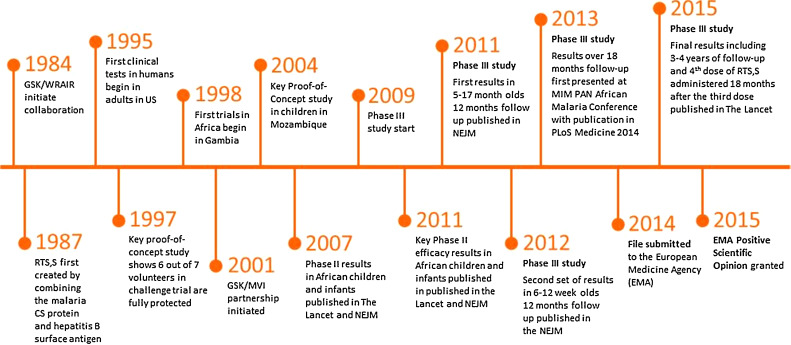
RTS,S was created in 1987 by scientists working at GSK laboratories. Early clinical development was conducted in collaboration with the Walter Reed Army Institute for Research. In January 2001, GSK and PATH’s Malaria Vaccine Initiative (PATH/MVI), with grant monies from the Bill & Melinda Gates Foundation to PATH, entered into a public-private partnership to develop RTS,S for infants and young children living in malaria-endemic regions in sub-Saharan Africa. RTS,S aims to trigger the immune system to defend against the first stages when the Plasmodium falciparum malaria parasite enters the human host’s bloodstream through a mosquito bite and infects liver cells. The vaccine is designed to prevent the parasite from infecting the liver, where it can mature, multiply, re-enter the bloodstream, and infect red blood cells, which can lead to disease symptoms.
Phase I and II clinical trials allowed an initial assessment of the candidate vaccine’s safety and efficacy profile, first in adult volunteers in the United States and Belgium, followed by adults, adolescents, children, and then infants living in malaria-endemic regions in Africa. Results of Phase II proof-of-concept trials in Mozambique, published in The Lancet in 2004 and 2007, demonstrated that it was possible to provide partial protection against malaria to African children and infants, respectively.1,2 The RTS,S Phase III efficacy and safety trial—the largest malaria vaccine trial in Africa to date—began in May 2009 and ended in early 2014. The trial involved 15,459 infants and young children at 11 sites in seven African countries (Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and Tanzania)
Developmental Period
Phase III Trial Results
Results of the study after a year of follow-up were published in the New England Journal of Medicine in November 2011 (for children aged 5-17 months) and December 2012 (for infants aged 6-12 weeks).3,4 These results showed that three doses of RTS,S reduced clinical malaria by approximately half in children 5-17 months of age at first vaccination. In a subsequent analysis after 18 months of follow up, children vaccinated with RTS,S experienced 46% fewer cases of clinical malaria, compared to children immunized with a comparator vaccine. 6,7 Efficacy waned over time. These results were achieved on top of existing malaria interventions, such as insecticide-treated bed nets, which were used by almost 80% of the trial participants.

Final study results, published in The Lancet in April 2015,7 includes analysis of vaccine efficacy, immunogenicity, safety, impact of RTS,S/AS01 over a median of 48 months of follow-up post-dose 1, and the effect of a fourth dose of vaccine.
Working of Mosquirix
The active substance in Mosquirix is made up of proteins found on the surface of the Plasmodium falciparum parasites and the hepatitis B virus.
When a child is given the vaccine, the immune system recognises the proteins from the parasite and virus as ‘foreign’ and makes antibodies against them. The immune system will then be able to produce antibodies more quickly when the child is naturally exposed to the malaria parasites and the hepatitis B virus in the future.
Mosquirix induces antibodies against malaria parasites that have entered the blood (via a mosquito bite) and have reached or are traveling to the liver, where they can mature and multiply. The vaccine thus limits the ability of the parasites to mature in the liver and cause clinical disease.
When is RTS,S coming to India?
In January this year, GSK, PATH and Bharat Biotech signed a product transfer agreement to help ensure the long-term supply of the RTS,S vaccine. However, experts The Indian Express spoke to feel there is no immediate “rush” to introduce it in India. Although malaria is a concern in India, the burden has reduced through interventions such as antimalarial drugs, mosquito nets and insecticide: from 1,018 deaths in 2010 to 93 in 2020.
Besides, the vaccine’s efficacy is modest. Officials with the National Malaria Control Programme said that a vaccine has to give protection of over at least 65%.
Pricing
A final price for RTS,S has not been determined; however, PATH, GSK, and other partners remain committed to helping ensure that RTS,S—if made available for widescale use—reaches the infants Page 4 and children who need it most. In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms. The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access. GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around five percent, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.
GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around five percent, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.
What other vaccines are in development?
Several are being tested, and at least one has shown promise. Called R21/Matrix M, this candidate vaccine showed an efficacy of 77% in phase 2 trials in May this year. R21/Matrix M is a modified version of Mosquirix, and has been developed by researchers at the University of Oxford. Lead researcher Adrian Hill, director of Jenner Institute and professor of vaccinology at Oxford University, had said he believed this vaccine was the first to reach WHO’s goal of at least 75% efficacy.
Dr V S Chauhan, former director of Delhi-based International Centre for Genetic Engineering and Biology, and known for his efforts to develop a recombinant malaria vaccine, said R21/Matrix M held a lot of promise. “This vaccine is definitely a big hope, but it still has to undergo phase 3 trials,” he said.

