
Hyloris announces FDA Acceptance Of New Drug Application for Maxigesic IV in Post-Operative Pain.
Hyloris Pharmaceuticals SA, a speciality biopharma company committed to addressing unmet medical needs through reinventing existing medications, announces that the U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for Maxigesic IV, a novel, unique combination of 1000mg paracetamol and 300mg ibuprofen solution for infusion, for the treatment of post-operative pain. In addition, the Company informs that the US Patent and Trademark Office (USPTO) has issued a Notice of Allowance and granted the process and formulation patents of Maxigesic IV in the U.S. The patents are expected to be issued early 2022 after completion of the final administrative requirements.
The FDA is expected to confirm the Prescription Drug User Fee Act (PDUFA) action date for the filing in due course. The PDUFA date – the date at which the FDA must respond to the application – will be notified later this year and is estimated to be between August and September 2022.
Stijn Van Rompay, Chief Executive Officer of Hyloris, commented: “The NDA acceptance marks an important milestone for our company, and is a major step toward bringing much needed innovation in non-opioid, post-operative pain management and addressing the current opioid crisis, which is responsible for many deaths in the U.S. each year. Together with our partners, we are looking forward to working with the FDA and to further executing on our global commercial rollout. Upon approval, Maxigesic IV will be commercialised by Hikma Pharmaceuticals, a leading supplier of complex, injectable hospital products in the U.S.”
The NDA submission is based on positive data from two Phase 3 studies of Maxigesic IV: i) a randomised, double-blind, placebo-controlled efficacy trial in 276 patients following bunionectomy surgery; and ii) an open-label, multi-centre, single arm, multiple dose safety study in 232 patients undergoing general, orthopaedic, or plastic surgery. As previously reported, treatment with Maxigesic IV was well-tolerated, had a faster onset of action and offered higher pain relief compared to ibuprofen IV or paracetamol IV alone in the same doses. Moreover, the superior analgesic effect of Maxigesic IV was supported by a range of secondary endpoints, including reduced opioid usage rates compared to the paracetamol IV, ibuprofen IV and placebo treatment groups (P<0.005).2 The open-label Phase 3 safety study demonstrated that Maxigesic IV, administered 6-hourly as a 15-minute infusion over an exposure period of 48 hours to 5 days, was safe and well-tolerated, and was perceived positively by study participants, supporting a favourable risk benefit profile.
Maxigesic IV has been developed under the collaboration agreement signed in 2012 between Hyloris and AFT Pharmaceuticals. The product is currently licensed in over 100 countries across the globe, has been registered in 28 countries and is now launched in 5 countries. Maxigesic IV is protected by several granted patents and pending patent applications.
What is Maxigesic used for?
Maxigesic® is used for temporary relief of pain associated with: headache, migraine headache, tension headache, sinus pain, toothache, dental procedures, backache, sore throat, arthritis, tennis elbow, period pain, muscular pain, rheumatic pain, aches and pains associated with colds and flu. Reduces fever.
Maxigesic® IV contains paracetamol, an analgesic medicine which relieves pain and reduces fever. Maxigesic® IV also contains ibuprofen, which belongs to a group of medicines called non-steroidal anti-inflammatory drugs (NSAIDs). Ibuprofen relieves pain, inflammation and fever.
Maxigesic® IV is a solution which is given by intravenous (IV) infusion directly into a vein, and is used to relieve pain or reduce fever. Your doctor may have prescribed Maxigesic® IV for another reason.
Pharmacodynamic properties Pharmacotherapeutic group:
Other Analgesics and Antipyretics, Anilides; ATC code: N02BE51.
Paracetamatol
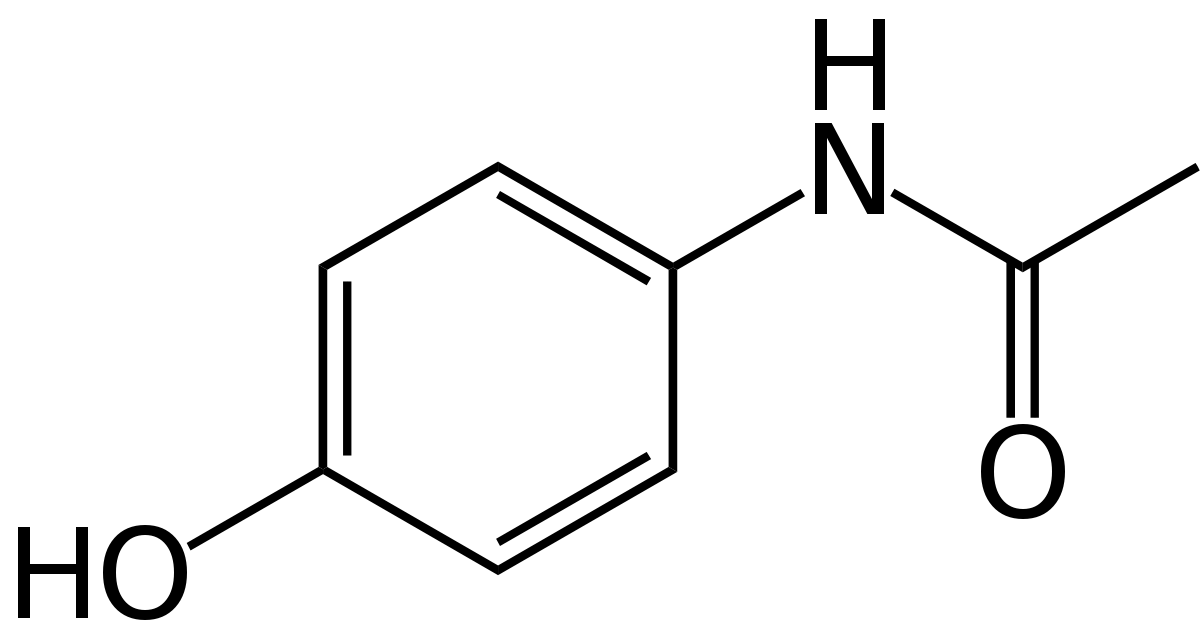
Structure of Paracetamol.
The chemical name for paracetamol is N-(4-hydroxyphenyl)acetamide.
It has the following structural formula:
Molecular formula: C8H9NO2
Molecular weight: 151.2
Solubility: Paracetamol is sparingly soluble in water, freely soluble in alcohol, and very slightly soluble in ether and methylene chloride. CAS number: 103-90-2
Ibuprofen
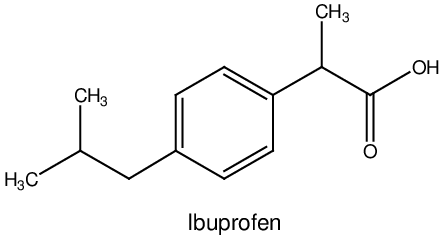
Structure of Ibuprofen
The chemical name for ibuprofen sodium dihydrate is (2RS)-2-[4-(2-methylpropyl)phenyl]propanoic acid sodium salt dihydrate. It has the following structural formula:
Molecular formula: C13H17O4Na.2H2O (sodium dihydrate salt)
Molecular weight: 206.3 (free acid), 228.3
(sodium dihydrate salt) pKa: 4.43 ± 0.03
Solubility: Ibuprofen is very slightly soluble in water
Pharmacodynamic effects:
In a phase III study in 276 patients with at least moderate pain following bunionectomy surgery, perceptible pain relief occurred within 10 minutes and meaningful pain relief occurred within 75 minutes following the administration of Maxigesic® IV. The peak analgesic effect was obtained at 4 hours, before the pain relief gradually declined to lower levels by 6 hours. Maxigesic® IV has not been studied in the reduction of fever; however, both paracetamol and ibuprofen have antipyretic properties.
Clinical efficacy and safety:
In a phase III efficacy study in 276 patients with at least moderate pain following bunionectomy surgery, the analysis of the primary endpoint, the time-adjusted Summed Pain Intensity Difference (SPID) 0-48 hours, demonstrated that Maxigesic® IV (mean=23.41, SE=2.50) provided more effective pain relief than placebo (mean= – 1.30, SE=3.07), paracetamol (mean=10.42, SE=2.50) or ibuprofen (mean= 9.51, SE=2.49), with a high level of statistical significance (p<0.001)
Contraindications:
Maxigesic® IV is contraindicate
- In patients with hypersensitivity to the active substances
- In patients with active alcoholism, as chronic excessive alcohol ingestion may predispose patients to paracetamol hepatoxicity
- in patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal anaphylactic-like reactions to NSAIDs have been reported in such patients
- for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surger
- in patients with impaired kidney function, impaired liver function, heart problems or heart failure;
- in patients with active gastrointestinal bleeding, peptic ulceration or other stomach disorders; in patients with spinal cord injuries;
- during pregnancy or in patients planning to become pregnant;
- during breastfeeding
It is recommended to use a suitable analgesic oral treatment as soon as this administration route is possible. In order to avoid the risk of overdose, check that other medicines administered do not contain paracetamol. Doses higher than the recommended entail a risk of very serious liver damage. Clinical symptoms and signs of liver damage are usually seen first after two days with a maximum usually after 4 to 6 days. Treatment with antidote should be given as soon as possible.
Special warnings and precautions for use:
Maxigesic® IV should be used with caution in cases of:
- Glucose 6 Phosphate Dehydrogenase (G6PD) deficiency (may lead to haemolytic anaemia)
- anorexia, bulimia or cachexia;
- chronic malnutrition (low reserves of hepatic glutathione),
- dehydration, hypovolemia
About Post-Operative Pain Management:
Globally, approximately 1.2 billion vials are sold per year in the non-opioid IV analgesic space, of which >260 million vials of IV paracetamol that represent a global market of >$700 million.4 In 2019, 51 million surgical procedures were performed in the U.S. and the overall treatment of post-operative pain has not substantially improved over the past 20 years, with the misuse of opioids remaining a key public health issue. Recently released data by the Centers for Disease Control and Prevention (CDC) show that drug overdose deaths reached a record high of 93,331 in 2020, of which 57,550 (62%) were due to synthetic opioid misuse. This represented a significant increase as compared to 2015 where synthetic opioids were involved in 18% of all overdose deaths. The CDC estimate that the total economic burden of prescription opioid misuse in the U.S. alone is $78.5 billion a year, including the costs of healthcare, lost productivity, addiction treatment, and criminal justice involvement.
About Hyloris Pharmaceuticals:
Hyloris is a specialty biopharma company focused on innovating, reinventing, and optimising existing medications to address important healthcare needs and deliver relevant improvements for patients, healthcare professionals and payors. Hyloris has built a broad, patented portfolio of 13 reformulated and repurposed value-added medicines that have the potential to offer significant advantages over available alternatives. Outside of its core strategic focus, the Company also has 3 high barrier generic products in development and registration phase. Two products are currently in initial phases of commercialisation with partners: Sotalol IV for the treatment of atrial fibrillation, and Maxigesic® IV, a non-opioid post-operative pain treatment. The Company’s development strategy primarily focuses on the FDA’s 505(b)2 regulatory pathway, which is specifically designed for pharmaceuticals for which safety and efficacy of the molecule have already been established. This pathway can reduce the clinical burden required to bring a product to market, and significantly shorten the development timelines and reduce costs and risks. Hyloris is based in Liège, Belgium.

