Summary :
Pfizer Inc. (plaintiff) filed a patent infringement suit against West-Coast Pharmaceutical works Ltd. (defendant) for inviting manufacturers to contract with Pfizer concerning the manufacturing and marketing of 𝗣𝗮𝗹𝗯𝗼𝗰𝗶𝗰𝗹𝗶𝗯 and 𝗖𝗿𝗶𝘇𝗼𝘁𝗶𝗻𝗶𝗯 compounds without obtaining any license from them.
Pfizer holds valid patents for compounds 𝗣𝗮𝗹𝗯𝗼𝗰𝗶𝗰𝗹𝗶𝗯 and 𝗖𝗿𝗶𝘇𝗼𝘁𝗶𝗻𝗶𝗯, administered to treat cancer. They also placed in record one communication received by one of their stockists from the defendant.

Patent infringement Case
Initially, the Court was hesitant to grant ex-parte interlocutory injunction taking into account that the drugs in question were 𝘢𝘯𝘵𝘪-𝘤𝘢𝘯𝘤𝘦𝘳 𝘥𝘳𝘶𝘨𝘴. But, considering that the defendants were proposing to release the drug in the market without obtaining a license from the plaintiff, the Court issued an interim injunction in the plaintiff’s favor.
Justice C. Hari Shankar of the Hon’ble High Court of Delhi restrained the defendants from releasing in the market, entering into contracts with manufacturers and manufacturing/marketing 𝗣𝗮𝗹𝗯𝗼𝗰𝗶𝗰𝗹𝗶𝗯 and 𝗖𝗿𝗶𝘇𝗼𝘁𝗶𝗻𝗶𝗯, or pharmaceutically acceptable salts thereof, in which Pfizer holds a valid suit patent till the next date of hearing.
Palbociclib
Palbociclib, sold under the brand name Ibrance among others, is a medication developed by Pfizer for the treatment of HR-positive and HER2-negative breast cancer. It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6. Palbociclib was the first CDK4/6 inhibitor to be approved as a cancer therapy.
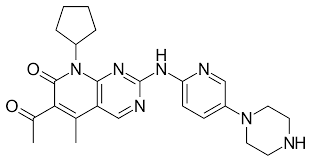
Structure of Palbociclib
Use in Cancer
Palbociclib is approved to be used with other drug to treat:
- Breast cancer that is hormone receptor positive (HR+) and HER2 negative (HER2-) and has spread.
- It is used with fulvestrant in adults whose cancer has gotten worse after treatment with hormone therapy.
- It is used with an aromatase inhibitor in postmenopausal women and in men who have not been treated with hormone therapy.
- Palbociclib is also being studied in the treatment of other types of cancer.
Breast Cancer
Indication
- Indicated for treatment of adults with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer as follows:
-
Men or postmenopausal women
- Use in combination with aromatase inhibitor as initial endocrine-based therapy
-
Patients with disease progression following endocrine therapy
- Use in combination with fulvestrant
Combination therapy with aromatase inhibitor
- Palbociclib 125 mg PO qDay for Days 1-21 of each 28-day cycle
- Aromatase inhibitor: See prescribing information
- Continue until disease progression or unacceptable toxicity
- Men treated with combination palbociclib plus aromatase inhibitor therapy, consider treatment with a luteinizing hormone-releasing hormone (LHRH) agonist according to current clinical practice standards
Combination therapy with fulvestrant
- Palbociclib 125 mg PO qDay for Days 1-21 of each 28-day cycle
- Fulvestrant 500 mg IM on Days 1, 15, and 29, and then once monthly thereafter
- Continue until disease progression or unacceptable toxicity
- Pre/perimenopausal women treated with the combination palbociclib plus fulvestrant should also be treated with an LHRH agonist according to current clinical practice standards
Mechanism of Action
Through inhibition of cyclin D-CDK4/6 complex activity, palbociclib inhibits the phosphorylation of retinoblastoma (Rb) protein, blocking cell cycle progression from G1 into S phase.
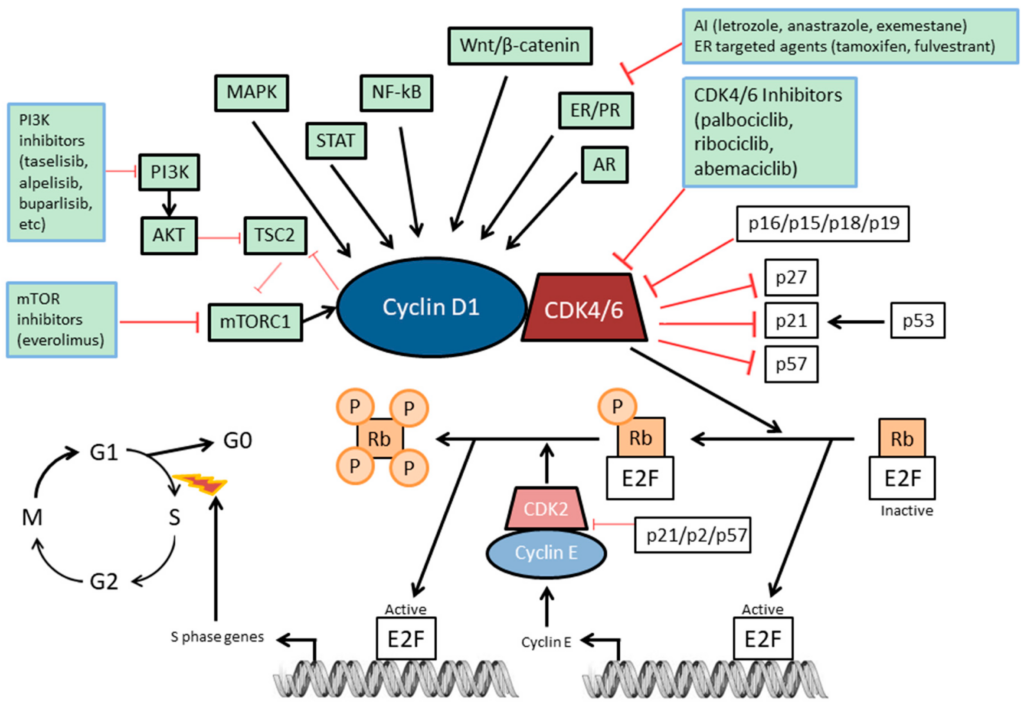
Palbociclib mechanism of action
CDK 4/6 inhibitors are a class of prescription medicines that are used in combination with hormone therapies to treat adults with hormone receptor (HR)-positive, human epidermal growth factor 2 (HER2)-negative advanced or metastatic breast cancer that has spread to other parts of the body. CDK 4/6 inhibitors block certain molecules involved in promoting the growth of cancer cells. FDA approved palbociclib in 2015, and both ribociclib and abemaciclib in 2017. CDK 4/6 inhibitors have been shown to improve the amount of time after the start of treatment the cancer does not grow substantially and the patient is alive, called progression-free survival (See List of FDA-Approved CDK 4/6 Inhibitors below).
Palbociclib may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- nausea
- diarrhea
- vomiting
- decreased appetite
- changes in taste
- tiredness
- numbness or tingling in your arms, hands, legs, and feet
- sores on the lips, mouth, or throat
- unusual hair thinning or hair loss
- dry skin
- rash
Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately or get emergency medical treatment:
- fever, chills, or signs of infection
- shortness of breath
- dizziness
- fast, irregular, or pounding heartbeat
- weakness
- unusual bleeding or bruising
- nosebleeds
Palbociclib may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
𝗖𝗿𝗶𝘇𝗼𝘁𝗶𝗻𝗶𝗯
Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication acting as an ALK and ROS1 inhibitor, approved for treatment of some non-small cell lung carcinoma
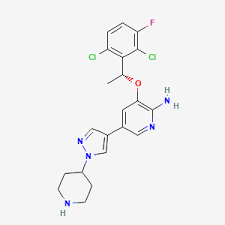
Structure of Crizotinib
Medical uses
Crizotinib is indicated for the treatment of metastatic non-small cell lung cancer (NSCLC) or relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-positive
Mechanism of action
Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c-Met), and Recepteur d’Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins.The formation of ALK fusion proteins results in the activation and dysregulation of the gene’s expression and signaling, which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrates concentration-dependent inhibition of ALK and c-Met phosphorylation in cell-based assays using tumor cell lines, and also demonstrates antitumor activity in mice bearing tumor xenografts that express EML4- or NPM-ALK fusion proteins or c-Met.
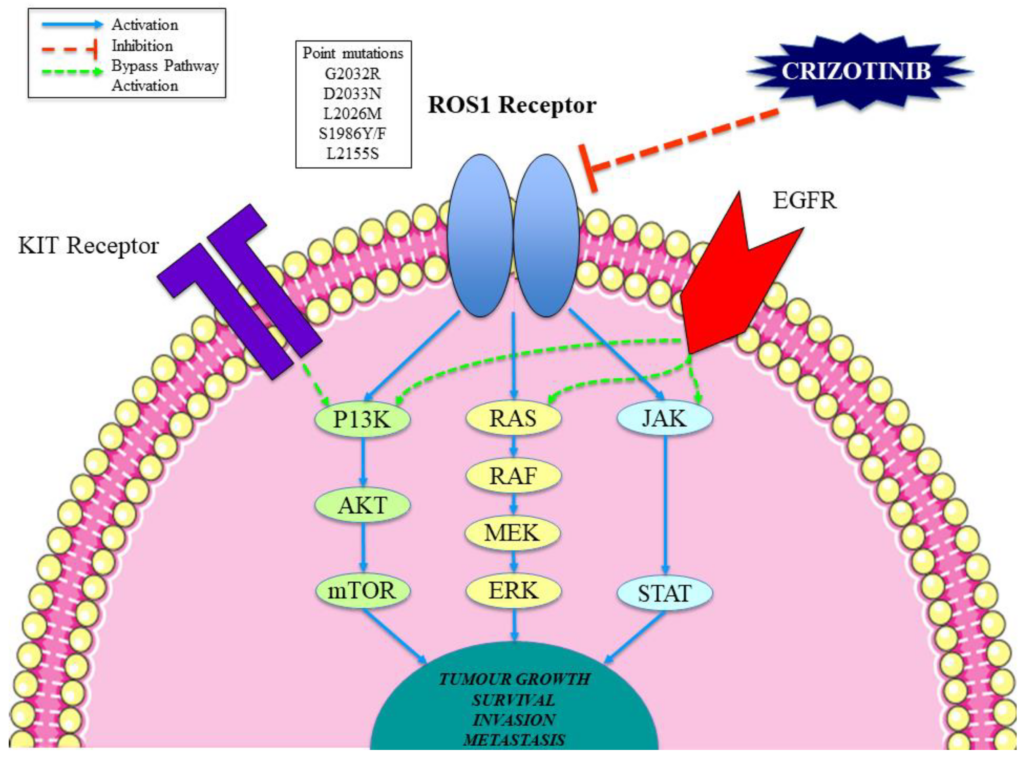
𝗖𝗿𝗶𝘇𝗼𝘁𝗶𝗻𝗶𝗯 mechanism of action
Crizotinib is a multitargeted small molecule tyrosine kinase inhibitor, which had been originally developed as an inhibitor of the mesenchymal epithelial transition growth factor (c-MET); it is also a potent inhibitor of ALK phosphorylation and signal transduction. This inhibition is associated with G1-S phase cell cycle arrest and induction of apoptosis in positive cells in vitro and in vivo. Crizotinib also inhibits the related ROS1 receptor tyrosine kinase.
Side Effects
Adverse events associated with the use of Xalkori for NSCLC may include, but are not limited to, the following:
- vision disorders
- nausea
- diarrhea
- vomiting
- edema
- constipation
- elevated transaminases
- fatigue
- decreased appetite
- upper respiratory infection
- dizziness
- neuropathy
Adverse events associated with the use of Xalkori for ALCL may include, but are not limited to, the following:
- diarrhea
- vomiting
- nausea
- vision disorder
- headache
- musculoskeletal pain
- stomatitis
- fatigue
- decreased appetite
- pyrexia
- abdominal pain
- cough
- pruritus
- Grade 3–4 laboratory abnormalities including neutropenia, lymphopenia, and thrombocytopenia
Indication 1 – for the treatment of ALK+ non-small cell lung cancer
Indication 2 – for pediatric patients 1 year of age and older and young adults with ALK+ relapsed or refractory, systemic anaplastic large cell lymphoma
Fact About Crizotinib and Palbociclib Exclusive Chemintelligence
Crizotinib (Xalkori ) :
– On August 24, 2011, the U.S. Food and Drug Administration approved crizotinib to treat certain late-stage non-small cell lung cancers.
– In October 2012, the European Medicines Agency (EMA) approved the use of crizotinib to treat non-small cell lung cancers that express the abnormal anaplastic lymphoma kinase (ALK) gene.
– In March 2016, the U.S. Food and Drug Administration approved crizotinib in ROS1-positive non-small cell lung cancer.
Palbociclib (Ibrance) :
It was developed by Pfizer for the treatment of HR-positive and HER2-negative breast cancer.
– November 2016 , the European Union was approved treatment for hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer either in combination with an aromatase inhibitor or, for women who have received prior endocrine therapy, in combination with fulvestrant.
– In March 2017, the FDA granted regular approval to palbociclib for hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative advanced or metastatic breast cancer, in combination with an aromatase inhibitor.
About West-Coast Pharmaceutical works Ltd
Established in the year 1965, ‘West Coast Pharmaceutical Works Limited’ has garnered a respectable name for itself in the domain of processing, supplying and exporting pharmaceutical products. Over the years, we have grown into a vast organization. Our experience and dedication makes us capable of processing all kinds of effective medicinal products.
Manufacturer of Pharmaceutical Products for various categories of products
1. Anti-Cancer Tablet & Capsules
2. Hormones Tablet & Capsules
3. Î’-Lactum Tablets (Coated / Uncoated)
4. Î’-Lactum Capsule & Dry Syrup
5. Oral Powder (ORS, PROTEIN POWDER)
6. Oral Liquid Section
7. General Tablet & Capsule
8. Disinfectant Products. (All hospital disinfectant products)
9. External Liquid/Ointment/Gel/Lotion/Nasal spray
10. Cosmetics products.(CREAM/ GEL/ LOTION/SOAPS/SHAMPOO/OILS)
11. Medicated Soaps
12. General Powder Repacking
13. Liquid Repacking
14. Dietary Supplement/Food Supplements/Nutraceutical products
About Pfizer
Pfizer Inc. is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German immigrants, Charles Pfizer and his cousin Charles F. Erhart.
Pfizer develops and produces medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company has several blockbuster drugs or products that each generate more than US$1 billion in annual revenues. In 2020, 52% of the company’s revenues came from the United States, 6% came from each of China and Japan, and 36% came from other countries.
Pfizer was a component of the Dow Jones Industrial Average stock market index from 2004 to August 2020. The company ranks 64th on the Fortune 500 and 49th on the Forbes Global 2000.


