KEYPOINTS:
- Pfizer Inc allow generic manufacturers to supply its experimental antiviral Covid-19 pill to 95 low- and middle-income countries. This will be done through a licensing agreement with international public health group Medicines Patent Pool.
- PAXLOVID™ (PF-07321332; ritonavir) was found to reduce the risk of hospitalization or death by 89% compared to placebo in non-hospitalized high-risk adults with COVID-19
- In the overall study population through Day 28, no deaths were reported in patients who received PAXLOVID™ as compared to 10 deaths in patients who received placebo
- Pfizer plans to submit the data as part of its ongoing rolling submission to the U.S. FDA for Emergency Use Authorization (EUA) as soon as possible
- India will be one of the beneficiary countries in the Pfizer deal.
Pfizer to allow Generic Versions of its Covid-19 Pill (Paxlovid) in 95 Countries
Pfizer Inc said on Tuesday, it will allow generic manufacturers to supply its experimental antiviral Covid-19 pill to 95 low- and middle-income countries. This will be done through a licensing agreement with international public health group Medicines Patent Pool. The voluntary licensing agreement between Pfizer and the MPP will allow the United Nations-backed group to grant sub-licenses to qualified generic drug manufacturers to make their own versions of PF-07321332. Pfizer will sell the pills it manufactures under the brand name Paxlovid.
The voluntary licensing agreement between Pfizer and the MPP will allow the United Nations-backed group to grant sub-licenses to qualified generic drug manufacturers to make their own versions of PF-07321332. Pfizer will sell the pills it manufactures under the brand name Paxlovid. Pfizer, which also makes one of the most widely used COVID-19 vaccines, has said the pill cut the chance of hospitalization or death for adults at risk of severe disease by 89 percent in its clinical trial. The drug will be used in combination with ritonavir, an HIV drug that is already available generically.
Pfizer’s licensing deal follows a similar arrangement by rival Merck & Co for generic manufacturing of its COVID-19 treatment. The deals are unusual arrangements that acknowledge the dire need for effective treatments as well as the pressure drugmakers are under to make their life-saving drugs accessible at very low costs.
“We are extremely pleased to have another weapon in our armory to protect people from the ravages of COVID-19,” Charles Gore, Executive Director of the Medicines Patent Pool, said in an interview.
Gore said he hoped the generic version of Pfizer’s drug will be available within months.
The 95 countries in the license agreement cover around 53 percent of the world’s population and include all low- and lower-middle-income countries and some upper-middle-income countries in Sub-Saharan Africa. They also include countries that have transitioned from lower-middle to upper-middle-income status in the past five years, Pfizer and the MPP said.
“We believe oral antiviral treatments can play a vital role in reducing the severity of COVID-19 infections… We must work to ensure that all people – regardless of where they live or their circumstances – have access to these breakthroughs,” Pfizer Chief Executive Albert Bourla said in a statement.
Pfizer will waive royalties on sales in low-income countries. It will also waive them in the other countries covered by the agreement as long as Covid-19 remains classified as a public health emergency of international concern by the World Health Organization.
Pfizer has said it will sell the drug using a tiered pricing approach based on the income level of each country.
In the United States, it expects to price its treatment close to where Merck has priced its drug at around $700 a course.
India will be one of the beneficiary countries in the Pfizer deal.
Pfizer’s version of the drug will be in high demand. The company has said it expects to manufacture 180,000 treatment courses by the end of next month and at least 50 million courses by the end of 2022.
Pfizer’s licensing deal follows a similar arrangement by rival Merck & Co for generic manufacturing of its Covid-19 treatment. The deals are unusual arrangements that acknowledge the dire need for effective treatments as well as the pressure drugmakers are under to make their life-saving drugs accessible at very low costs.
We believe oral antiviral treatments can play a vital role in reducing the severity of Covid-19 infections… We must work to ensure that all people – regardless of where they live or their circumstances – have access to these breakthroughs
Pfizer Chief Executive Albert Bourla, Pfizer will waive royalties on sales in low-income countries. It will also waive them in the other countries covered by the agreement as long as Covid-19 remains classified as a public health emergency of international concern by the World Health Organization.
Pfizer’s oral treatment is meant to be taken at home as a five-day regimen of 30 pills. Ten of the pills are a low dose of an HIV drug known as ritonavir, meant to slow the breakdown of Pfizer’s pill so that it remains active in the body longer.
What is Paxlovid (PF-07321332)?
PF-07321332 is an antiviral drug developed by Pfizer which acts as an orally active 3CL protease inhibitor. It is a covalent inhibitor, binding directly to the catalytic cysteine (Cys145) residue of the enzyme.
The PF-07321332/ritonavir combination drug is in phase III trials for the treatment of COVID-and will be sold under the trade name Paxlovid. In this combination, ritonavir serves to slow down metabolism of PF-07321332 by cytochrome enzymes to maintain higher circulating concentrations of the main drug. In November 2021, Pfizer announced positive phase 2/3 results, including 89% reduction in hospitalizations when given within three days after symptom onset. Despite not being fully approved yet in either country, the UK has begun stockpiling PF-07321332, and Australia has preordered 500,000 courses of the drug.
Chemistry:
Full details of the synthesis of PF-07321332 were first published by scientists from Pfizer
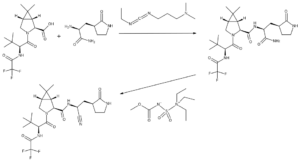
Chemistry of PF-07321332
In the penultimate step, a synthetic homochiral amino acid is coupled with a homochiral amino amide using the water-soluble carbodiimide EDCI as dehydrating agent. The resulting intermediate is then treated with Burgess reagent, which dehydrates the amide group to the nitrile of the product.
About the Phase 2/3 EPIC-HR Study Interim Analysis:
The primary analysis of the interim data set evaluated data from 1219 adults who were enrolled by September 29, 2021. At the time of the decision to stop recruiting patients, enrollment was at 70% of the 3,000 planned patients from clinical trial sites across North and South America, Europe, Africa, and Asia, with 45% of patients located in the United States. Enrolled individuals had a laboratory-confirmed diagnosis of SARS-CoV-2 infection within a five-day period with mild to moderate symptoms and were required to have at least one characteristic or underlying medical condition associated with an increased risk of developing severe illness from COVID-19. Each patient was randomized (1:1) to receive PAXLOVID™ or placebo orally every 12 hours for five days.
About the Phase 2/3 EPIC-HR Study Safety Data:
The review of safety data included a larger cohort of 1881 patients in EPIC-HR, whose data were available at the time of the analysis. Treatment-emergent adverse events were comparable between PAXLOVID™ (19%) and placebo (21%), most of which were mild in intensity. Among the patients evaluable for treatment-emergent adverse events, fewer serious adverse events (1.7% vs. 6.6%) and discontinuation of study drug due to adverse events (2.1% vs. 4.1%) were observed in patients dosed with PAXLOVID™ compared to placebo, respectively.
About PAXLOVID™ (PF-07321332; ritonavir) and the EPIC Development Program:
PAXLOVID™ is an investigational SARS-CoV-2 protease inhibitor antiviral therapy, specifically designed to be administered orally so that it can be prescribed at the first sign of infection or at first awareness of an exposure, potentially helping patients avoid severe illness which can lead to hospitalization and death. PF-07321332 is designed to block the activity of the SARS-CoV-2-3CL protease, an enzyme that the coronavirus needs to replicate. Co-administration with a low dose of ritonavir helps slow the metabolism, or breakdown, of PF-07321332 in order for it to remain active in the body for longer periods of time at higher concentrations to help combat the virus.
PF-07321332 inhibits viral replication at a stage known as proteolysis, which occurs before viral RNA replication. In preclinical studies, PF-07321332 did not demonstrate evidence of mutagenic DNA interactions.
Pfizer initiated the EPIC-HR study in July 2021 following positive Phase 1 clinical trial results and continues to evaluate the investigational antiviral in additional EPIC studies. In August 2021, Pfizer initiated the Phase 2/3 EPIC-SR (Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients), to evaluate efficacy and safety in patients with a confirmed diagnosis of SARS-CoV-2 infection who are at standard risk (i.e., low risk of hospitalization or death). EPIC-SR includes a cohort of vaccinated patients who have an acute breakthrough symptomatic COVID-19 infection and who have risk factors for severe illness. In September, Pfizer initiated the Phase 2/3 EPIC-PEP (Evaluation of Protease Inhibition for COVID-19 in Post-Exposure Prophylaxis) to evaluate efficacy and safety in adults exposed to SARS-CoV-2 by a household member.
About Pfizer’s Commitment to Equitable Access:
Pfizer is committed to working toward equitable access to PAXLOVID™ for all people, aiming to deliver safe and effective antiviral therapeutics as soon as possible and at an affordable price. If our candidate is successful, during the pandemic, Pfizer will offer our investigational oral antiviral therapy through a tiered pricing approach based on the income level of each country to promote equity of access across the globe. High and upper-middle income countries will pay more than lower income countries. The company has entered into advance purchase agreements with multiple countries and is in negotiations with several others. Pfizer has also begun and will continue to invest up to approximately $1 billion to support the manufacturing and distribution of this investigational treatment, including exploring potential contract manufacturing options to help ensure access across low- and middle-income countries, pending regulatory authorization.
The company is working to ensure access for its novel antiviral candidate for those most in need around the world, pending successful trial results and regulatory approval.
About Pfizer: Breakthroughs That Change Patients’ Lives:
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products, including innovative medicines and vaccines. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world’s premier innovative biopharmaceutical companies, we collaborate with health care providers, governments and local communities to support and expand access to reliable, affordable health care around the world. For more than 150 years, we have worked to make a difference for all who rely on us.
About Pfizer:
Pfizer Inc. is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German immigrants, Charles Pfizer (1824-1906) and his cousin Charles F. Erhart (1821-1891).
Pfizer develops and produces medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company has several blockbuster drugs or products that each generate more than US$1 billion in annual revenues. In 2020, 52% of the company’s revenues came from the United States, 6% came from each of China and Japan, and 36% came from other countries.
Pfizer was a component of the Dow Jones Industrial Average stock market index from 2004 to August 2020. The company ranks 64th on the Fortune 500and 49th on the Forbes Global 2000.
SOURCE: https://timesofindia.indiatimes.com/world/rest-of-world/pfizer-to-allow-generic-versions-of-its-covid-19-pill-in-95-countries/articleshow/87739077.cms
For more Information: Sign-in Websites for Agrochemical & Pharmaceutical Databases:
Website: https://www.chemrobotics.com/ (Agrochemical Databases)
Website: https://chemroboticspharma.com/ (Pharmaceutical Databases)


