Summary –
- A new drug recently introduced for use in the treatment of refractory/relapsed multiple myeloma looks like it will also find a role in the treatment of patients with newly diagnosed transplant-eligible multiple myeloma.
- The drug is isatuximab (Sarclisa, Sanofi), an anti-CD38 antibody that was approved last year for use in patients with advanced disease.
- It was developed by ImmunoGen and Sanofi-Aventis with the development name SAR-650984.
- It was granted orphan drug designation for multiple myeloma by the European Medicines Agency (EMA) in April 2014, and by the U.S. Food and Drug Administration (FDA) in December 2016
- It was approved for medical use in the European Union in May 2020
Patients with newly diagnosed, transplant-eligible multiple myeloma (MM) who received isatuximab (Sarclisa) combined with standard treatment as induction therapy were more likely to achieve minimal residual disease (MRD) negativity than patients treated with standard therapy alone, a researcher reported.
In the GMMG-HD7 trial, half of the patients who received the anti-CD38 monoclonal antibody combined with RVd — lenalidomide (Revlimid), bortezomib (Velcade) and dexamethasone — achieved MRD negativity after induction therapy versus about a third of patients receiving RVd only, according to Hartmut Goldschmidt, MD, of the Heidelberg University Hospital and National Center of Tumor Diseases Heidelberg in Germany.

Sanofi’s Isatuximab Added to RVd Boosts Response in Multiple Myeloma
“The GMMG-HD7 trial is the first phase III trial powered to evaluate MRD negativity at the end of induction in transplant-eligible, newly diagnosed multiple myeloma patients,” Goldschmidt said at a press briefing at the American Society of Hematology (ASH) annual meeting.
He added that the MRD-negativity rate achieved with the isatuximab-RVd combination “is the highest described to date in a randomized setting.”
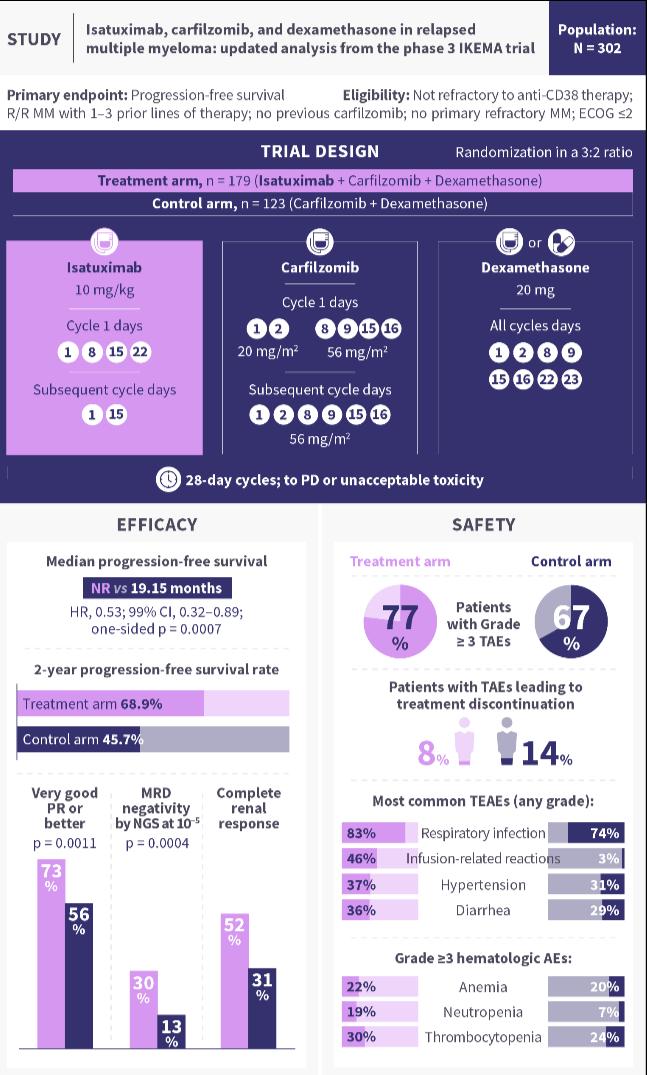
In March 2021, the FDA approved isatuximab in combination with carfilzomib (Kyprolis) and dexamethasone for the treatment of adult patients with relapsed or refractory MM who have received one to three prior lines of therapy, based on results from the IKEMA trial.
The GMMG-HD7 trial (done from 2018-2020) included 662 patients from 67 sites in Germany who were randomized to receive three 42-day cycles of RVd alone, or in combination with isatuximab. The primary endpoint of the trial was MRD negativity assessed by next-generation flow after induction, with secondary endpoints including rates of complete response (CR) after induction, and safety. Of the 662 patients, 660 were included in the intent-to-treat analysis, and 658 were eligible for induction. Data cut-off for the current analysis was April 2021.
MRD-negativity rates after induction were 35.6% for RVd alone compared with 50.1% for the RVd/isatuximab combination (odds ratio 1.83, 95% CI 1.34-2.51, P<0.001).
On multivariate analyses (including treatment arm, performance status, renal impairment, age, and sex), treatment with isatuximab-RVd, compared with RVd alone, remained the only significant predictor for increased MRD negativity after induction (OR 1.82, 95% CI 1.33-2.49, P<0.001).
There was no difference in CR rates after induction between the RVd and isatuximab-RVd arms (21.6% vs. 24.2%, P=0.46). However, the investigators observed that the rate of very good partial response or better was significantly higher in the isatuximab-RVd arm (60.5% vs 77.3%, P<0.001). The rates of progressive disease were 4.0% in the RVd arm and 1.5% in the isatuximab-RVd cohort.
As for adverse events, “the addition of isatuximab had no impact on the safety profile or dose intensity of the RVd backbone,” Goldschmidt reported.
He reported that at least one grade ≥3 adverse event (AE) on induction occurred in 61.3% of patients in the RVd cohort, and 63.6% in the RVd-isatuximab group. Rates of serious AEs on induction were similar between RVd and isatuximab-RVd (36.3% vs. 34.8%, P=0.75), while eight and four patients died during induction in the RVd and isatuximab-RVd groups, respectively.
As expected, rates of leukocytopenia and neutropenia were higher when isatuximab was added to RVd (26.4% vs 9.1% for RVd alone), “but this did not translate into increased rates of infection during induction treatment,” Goldschmidt said.
The investigators observed that fewer patients discontinued treatment with isatuximab-RVd than with RVd alone, while the addition of the anti-CD38 monoclonal antibody had no impact on RVd-dose intensity.
When asked about the clinical relevance of MRD as an endpoint, Goldschmidt noted that advances in technology for evaluating MRD in myeloma have improved significantly over the last decade. He also pointed out there are ongoing discussions between the International Myeloma Working Group and the FDA about the acceptance of MRD in bone marrow in myeloma patients as an endpoint in clinical trials.
“I think the trial is a tremendous design for someone [like me] who treats more non-Hodgkin lymphoma,” said press briefing moderator Laurie S. Sehn, MD, MPH, of the University of British Columbia in Vancouver. “We’re desperately looking for surrogate markers that allow us to speed up the answers to clinical trials, and I think using MRD in myelomas is quickly becoming a very important surrogate marker.”
As expected, rates of leukocytopenia and neutropenia were higher when isatuximab was added to RVd (26.4% vs 9.1% for RVd alone), “but this did not translate into increased rates of infection during induction treatment,” Goldschmidt said.
The investigators observed that fewer patients discontinued treatment with isatuximab-RVd than with RVd alone, while the addition of the anti-CD38 monoclonal antibody had no impact on RVd-dose intensity.
When asked about the clinical relevance of MRD as an endpoint, Goldschmidt noted that advances in technology for evaluating MRD in myeloma have improved significantly over the last decade. He also pointed out there are ongoing discussions between the International Myeloma Working Group and the FDA about the acceptance of MRD in bone marrow in myeloma patients as an endpoint in clinical trials.
“I think the trial is a tremendous design for someone [like me] who treats more non-Hodgkin lymphoma,” said press briefing moderator Laurie S. Sehn, MD, MPH, of the University of British Columbia in Vancouver. “We’re desperately looking for surrogate markers that allow us to speed up the answers to clinical trials, and I think using MRD in myelomas is quickly becoming a very important surrogate marker.”
“As we know, these patients with myeloma get these sequences of therapy, and its hard to pick out what component of the package is making an impact at each part of the treatment,” she said, adding that “Being able to isolate out the importance of the induction regimen using MRD as an early surrogate is a very important design, and serves to demonstrate the utility isatuximab is adding to that regimen.”
Goldschmidt noted that other studies presented at the ASH meeting will contain data on the importance of sustained MRD negativity, and observed that the GMMG-HD7 trial will evaluate whether MRD negativity is sustained with maintenance therapy. “We expect it will be a very good marker for the prognosis of myeloma patients,” he stated.
About Isatuximab
Isatuximab, sold under the brand name Sarclisa, is a monoclonal antibody (mAb) medication for the treatment of multiple myeloma.

The most common side effects include neutropenia (low levels of neutrophils, a type of white blood cell), infusion reactions, pneumonia (infection of the lungs), upper respiratory tract infection (such as nose and throat infections), diarrhoea and bronchitis (inflammation of the airways in the lungs)
Isatuximab is an anti-CD38 mAb intended to treat relapsed or refractory multiple myeloma. It entered in Phase II trials for multiple myeloma and T-cell leukemia in 2015
Medical Uses
In the United States it is indicated, in combination with pomalidomide and dexamethasone, for the treatment of adults with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor. It is also indicated in combination with carfilzomib and dexamethasone, for the treatment of adults with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy.
In the European Union it is indicated, in combination with pomalidomide and dexamethasone, for the treatment of adults with relapsed and refractory multiple myeloma (MM) who have received at least two prior therapies including lenalidomide and a proteasome inhibitor (PI) and have demonstrated disease progression on the last therapy.
History
It was granted orphan drug designation for multiple myeloma by the European Medicines Agency (EMA) in April 2014, and by the U.S. Food and Drug Administration (FDA) in December 2016.
A Phase III combination trial for plasma cell myeloma is comparing pomalidomide and dexamethasone with and without isatuximab is in progress with an estimated completion date of 2021.
In March 2020, it was approved for medical use in the United States.
The U.S. Food and Drug Administration (FDA) approved isatuximab-irfc in March 2020, based on evidence from a clinical trial (NCT02990338) of 307 subjects with previously treated multiple myeloma. The trial was conducted at 102 sites in Europe, North America, Asia, Australia and New Zealand.
It was approved for medical use in the European Union in May 2020.
Available forms
Isatuximab or isatuximab-irfc is available as a drug in an intravenous infusion form. Injection doses are 100 mg/5 mL (20 mg/mL) solution in single-dose vial or 500 mg/25 mL (20 mg/mL) solution in single-dose vial.
Mechanism of action
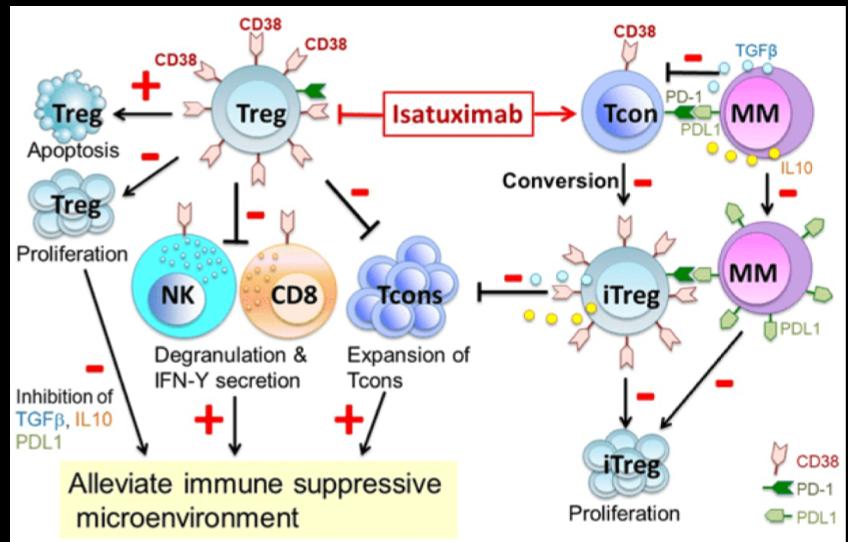
Cancer of the blood that is distinguished by an overproduction of malignant plasma cells in the bone marrow is called multiple myeloma. The myeloma cells are marked with uniformed overexpression of CD38 surface glycoproteins. Although these proteins are also expressed on other myeloid and lymphoid cells, the extent is relatively minor compared to myeloma cells. The fact that CD38 glycoproteins carry out various important cellular functions, and that they are plentiful on the surface of myeloma cells, has made them an appealing target for multiple myeloma treatment. CD38 was first described as an activation marker, but later the molecule displayed functions in adhesion to endothelial CD31 proteins, e.g. as an aiding component of the synapse complex, as well as an ectoenzyme implicated in the metabolism of extracellular NAD+ and cytoplasmic NADP. The tumour cells can evade the immune system, possibly due to adenosine, an immunosuppressive molecule that arises as a product of the ectoenzymatic activity of CD38.
Mechanism of Action of Isatuximab
Isatuximab-irfc is an IgG1-derived monoclonal antibody that selectively binds to the CD38 that exists on the exterior of hematopoietic and multiple myeloma cells (as well as other tumor cells). This drug induces apoptosis of tumor cells and activates immune effector mechanisms such as complement dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent cell-mediated cytotoxicity (ADCC). Isatuximab-irfc is able to stimulate natural killer (NK) cells in the absence of CD38-positive target tumor cells and blocks CD38-positive T-regulatory cells. Furthermore, the NADase activity of CD38 is adjusted by isatuximab, similarly to other CD38 antibodies. Contrarily to daratumumab however, isatuximab can incite apoptosis directly without cross-linking, and in its binding epitope.
According to the FDA, isatuximab-irfc alone has reduced ADCC and direct tumor cell killing activity in vitro in comparison to when it is combined with pomalidomide. As well as increased anti-tumor activity as opposed to isatuximab-irfc or pomalidomide only in a human multiple myeloma xenograft model.
Side effects
Adverse reactions to isatuximab-irfc may include neutropenia, infusion-related reactions and/or secondary primary malignancies. Of these three the most commonly occurring ones are the infusion-related reactions. Examples of the most frequent symptoms of infusion-related reactions are dyspnea, cough, chills, and nausea, while the severest signs and symptoms included hypertension and dyspnea.

