Synopsis:
- AbbVie’s blockbuster hopeful Rinvoq notched a win in a tricky-to-treat group of Crohn’s disease patients.
- The trial studied the AbbVie drug in certain patients with moderate-to-severe Crohn’s disease, and the results came after 12 weeks of treatment with the medicine or placebo.
- If Rinvoq passes muster at the FDA, it would become the first JAK inhibitor approved for the disease, opening the door to some $1.3 billion in peak Crohn’s disease sales by 2030, Datamonitor Healthcare predicts.
AbbVie’s blockbuster hopeful Rinvoq notched a win in a tricky-to-treat group of Crohn’s disease patients, potentially adding another arrow to the company’s post-Humira quiver.
The JAK inhibitor helped a “significantly” greater proportion of patients achieve clinical remission and endoscopic response than placebo did, phase 3 data from the company’s U-EXCEED study show. The trial studied the AbbVie drug in certain patients with moderate-to-severe Crohn’s disease, and the results came after 12 weeks of treatment with the medicine or placebo.
AbbVie expects to submit its Rinvoq application for Crohn’s disease in 2022. If Rinvoq passes muster at the FDA, it would become the first JAK inhibitor approved for the disease, opening the door to some $1.3 billion in peak Crohn’s disease sales by 2030, Datamonitor Healthcare predicts.

AbbVie’s Rinvoq Wins Phase Trail for Chron’s Disease
In the U-EXCEED study, investigators measured clinical remission using the Crohn’s Disease Activity Index (CDAI), plus patient-reported symptoms of stool frequency and abdominal pain (SF/AP). After a dozen weeks, 39% percent of patients who received the daily dose of Rinvoq 45 mg achieved clinical remission on the CDAI, versus 21% of placebo patients. Similar results were drawn from patient responses, too, with 40% of Rinvoq patients hitting remission per SF/AP, compared to 14% in the control arm.
Investigators also measured patient improvement in the intestinal mucosa by endoscopy. At 12 weeks, 35% of Rinvoq patients achieved endoscopic response, versus 4% in the placebo cohort.
Rinvoq’s wins didn’t stop there: Among patients taking corticosteroids at the trial’s start, significantly more on the JAK inhibitor achieved steroid-free clinical remission per CDAI and SF/AP versus those on placebo, AbbVie said.
AbbVie’s study enrolled moderate to severe Crohn’s disease patients who had an inadequate response or who couldn’t tolerate biologic treatments. More than 60% of patients in the trial had previously tried and failed on two or more biologics, AbbVie noted.
As the company’s best-selling drug Humira inches toward a 2023 patent cliff, AbbVie has pinned its growth hopes on its next-gen offerings Rinvoq and the IL-23 inhibitor Skyrizi. Still, Rinvoq’s fate—and that of its oral JAK inhibitor classmates—remains in flux, thanks to recent classwide safety warnings and restrictions.
The FDA this week updated the labels for AbbVie’s Rinvoq, Pfizer’s Xeljanz and Eli Lilly’s Olumiant to include safety warnings about serious heart-related side effects and cancer risks.
Importantly, the update includes a caveat that the drugs can only be used in patients who can’t tolerate or don’t respond well to classic TNF inhibitors like AbbVie’s Humira. For its Crohn’s trial, AbbVie is already studying Rinvoq in this specific patient population.
Safety in U-EXCEED was on par with Rinvoq’s other studies, with no new risks cropping up, AbbVie said.
AbbVie is expecting annual sales of about $8 billion for Rinvoq by 2025. The drug generated $453 million in the third quarter, and it amassed around $1.13 billion in worldwide sales for the first nine months of the year, placing it comfortably within the bounds of blockbuster territory.
About Rinvoq
Rinvoq (upadacitinib) is a prescription medicine that is a Janus kinase (JAK) inhibitor.
Rinvoq is used to treat adults with moderate to severe rheumatoid arthritis in patients that could not tolerate methotrexate or if treatment with methotrexate did not work well.
It is not known if Rinvoq is safe and effective in children under 18 years of age.
Rinvoq is available as a 15 mg extended-release tablet. The tablets are purple, biconvex oblong and imprinted with ‘a15’ on one side.
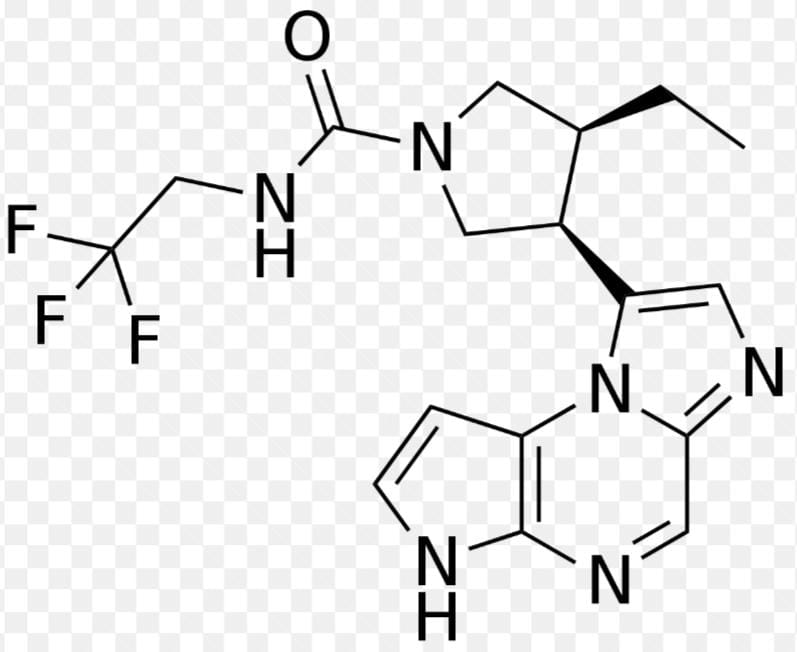
Structure of Rinvoq
Rinvoq side effects
Some people taking Rinvoq have developed heart attacks, strokes, or serious blood clots. Stop taking Rinvoq and seek emergency medical attention if you have:
- sudden shortness of breath;
- chest pain or pressure that may spread to your jaw, shoulder, arms, or back;
- nausea, Vomiting cold sweat;
- a light-headed feeling, like you might pass out;
- weakness on one side of your body;
- slurred speech, drooping on one side of your mouth; or
- pain, swelling, or redness in an arm or a leg.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor’s approval.
Other medications can affect the removal of Upadacitinib from your body, which may affect how Upadacitinib works. Examples include rifampin, phenytoin, among others.
About AbbVie :
AbbVie is an American publicly traded biopharmaceutical company founded in 2013. It originated as a spin-off of Abbott Laboratories.
On October 19, 2011, Abbott Laboratories announced its plan to separate into two publicly traded companies. The new Abbott Laboratories would specialize in diversified products including medical devices, diagnostic equipment and nutrition products, while AbbVie would operate as a research-based pharmaceutical manufacturer. The separation was effective January 1, 2013, and AbbVie was officially listed on the New York Stock Exchange (ABBV) on January 2, 2013.
According to Miles White, CEO at the time, the purpose of the split was to allow markets to value the two businesses separately.Some investors were concerned that the split was done to protect the value of the device business from the loss of value facing the drug division due to the imminent expiration of patents on Humira, which accounted for about half of the drug division’s revenue.
In June 2021, the US Senate Finance Committee, under Chair Ron Wyden (D-OR) began an investigation into the company probing whether the company used the Tax Cuts and Jobs Act of 2017 to buy back its own stock using income saved by the tax law’s providing billions in tax savings. Wyden, in a letter to AbbVie CEO Richard Gonzalez, pointed out that the company disclosed it suffered a 2020 pretax loss in the US of $4.5 billion and an overseas pretax profit of $7.9 billion the same year. Wyden accused the company of shifting revenue to avoid US taxes.

