Summary-
The Indian Pharmacopoeia Commission (IPC) has added 9 new IP impurities standards which includes Bromhexine Hydrochloride Impurity C, Cabergoline Impurity A, Cilostazol Impurity B, Citalopram Impurity B, Fosinopril Impurity A, o-toluene sulfonamide, p-toluene sulfonamide, Tributyl o-acetyl citrate/Acetyl Tributyl citrate, Vildagliptin Impurity D.
The Indian Pharmacopoeia Commission (IPC) has added nine new IP impurities standards, which include:
- Bromhexine Hydrochloride Impurity C,
- Cabergoline Impurity A,
- Cilostazol Impurity B,
- Citalopram Impurity B,
- Fosinopril Impurity A,
- O-toluene sulfonamide,
- P-toluene sulfonamide,
- Tributyl o-acetyl citrate/Acetyl Tributyl citrate,
- Vildagliptin Impurity D.
Impurity standards are used to perform the system suitability, qualitative and quantitative parameters for compliance to Indian Pharmacopoeia monograph.
The commission stated that certain monographs require the use of a chemical reference substance or a biological reference preparation or a reference spectrum.

Indian Pharmacopoeia Commission Add 9 Impurities
IPC in collaboration with USP also conducted a webinar on “Fundamentals of Dissolution” as a part of Capability Building initiative. The webinar was conducted “exclusively” for MSME (Micro, small and medium enterprises).
The session conducted by Dr. Rajeev Singh Raghuvanshi – Secretary-cum-Scientific Director, IPC and Josan Thomas, Scientific Liaison, USP India, covered the science behind dissolution, fundamentals of dissolution testing, and specifics related to the qualification of USP apparatus 1 and apparatus 2. The session saw great response with 339 participants from 149 unique companies.
The panelists for webinar includes
- Dr. Rajeev Singh Raghuvanshi – Secretary-cum-Scientific Director, IPC,
- Dr. Robin Kumar – Principal Scientific Officer,
- Dr. Gaurav Pratap Singh Jadaun – Senior Scientific Officer, IPC,
- Josan Thomas, Scientific Liaison, USP India,
- Mr. Girish Kapur- Vice President, USP India,
- Joseph Eaton – Senior Manager, USP Rockville.
The vision of IPC is to promote the highest standards of drugs for use in human and animals within practical limits of the technologies available for manufacture and analysis.
IPC is an autonomous institution of the ministry of health and family welfare, Government of India. IPC is created to set standards of drugs in the country. Its basic function is to update regularly the standards of drugs commonly required for treatment of diseases prevailing in this region. The mandate of the commission is to perform, inter-alia, functions such as revision and publication of the Indian Pharmacopoeia and National Formulary of India on a regular basis besides providing IP reference substances and training to the stakeholders on Pharmacopoeial issues.
Impurity
As defined by the United States Pharmacopeial (USP), impurity is “any component of a drug substance that is not the chemical entity defined as the drug substance and in addition, for a drug product, any component that is not a formulation ingredient
Bromhexine Hydrochloride Impurity C
Structure –
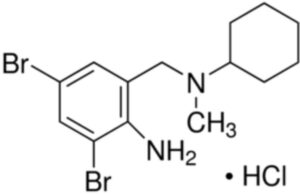
Bromhexine Hydrochloride is the hydrochloride salt form of bromhexine, a secretolytic, with mucolytic activity. Upon administration, bromhexine increases lysosomal activity and enhances hydrolysis of acid mucopolysaccharide polymers in the respiratory tract. This increases the production of serous mucus in the respiratory tract, which makes the phlegm thinner and decreases mucus viscosity. This contributes to its secretomotoric effect, and allows the cilia to more easily transport the phlegm out of the lungs. This clears mucus from the respiratory tract and may aid in the treatment of respiratory disorders associated with abnormal viscid mucus, excessive mucus secretion and impaired mucus transport.
Cabergoline Impurity A
Structure –
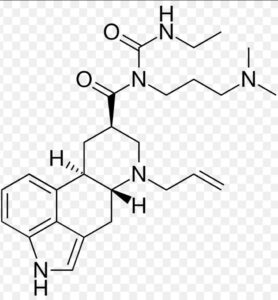
Cabergoline is a dopaminergic medication used in the treatment of high Prolactin levels, Prolactinomas, Parkinson’s disease, and for other indications. It is taken by mouth.
Cabergoline is an ergot derivative and a potent dopamine D2 receptor agonist.
Cabergoline was patented in 1980 and approved for medical use in 1993
Cilostazol Impurity B
Structure –
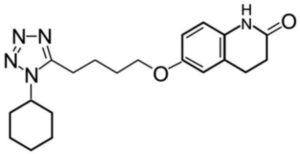
It is a medication used to help the symptoms of intermittent claudication in peripheral vascular disease. If no improvement is seen after 3 months, stopping the medication is reasonable. It may also be used to prevent stroke. It is taken by mouth.
Common side effects include headache, diarrhea, dizziness, and cough. Serious side effects may include decreased survival in those with heart failure, low platelets, and low white blood cells. Cilostazol is a phosphodiesterase 3 inhibitor which works by inhibiting platelet aggregation and dilating arteries.
Citalopram Impurity B
Structure –
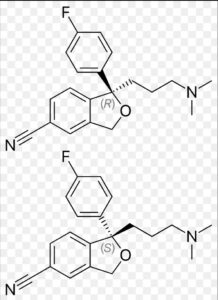
Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and social phobia. The antidepressant effects may take one to four weeks to occur. It is taken by mouth.
Common side effects include nausea, trouble sleeping, sexual problems, shakiness, feeling tired, and sweating. Serious side effects include an increased risk of suicide in those under the age of 25, serotonin syndrome, glaucoma, and QT prolongation. It should not be used in persons who take or have recently taken a MAO inhibitor. Antidepressant discontinuation syndrome may occur when stopped. There are concerns that use during pregnancy may harm the fetus
Fosinopril Impurity A
Structure –
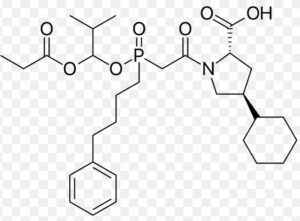
Fosinopril is used alone or in combination with other medications to treat high blood pressure. It is also used in combination with other medications to treat heart failure. Fosinopril is in a class of medications called angiotensin-converting enzyme (ACE) inhibitors. It works by decreasing certain chemicals that tighten the blood vessels, so blood flows more smoothly and the heart can pump blood more efficiently.
It is marketed, by Bristol-Myers Squibb under the trade name Monopril. Fosinopril is a cascading pro-drug. The special niche for the medication that differentiates it from the other members of the ACE Inhibitor drug class is that was specifically developed for the use for patients with renal impairment. This was through manipulation of the metabolism and excretion, and is seen that fifty percent of the drug is hepatobiliary cleared, which can compensate for diminished renal clearance. The remaining fifty percent is excreted in urine. It does not need dose adjustment.
O-toluene sulfonamide
Structure –
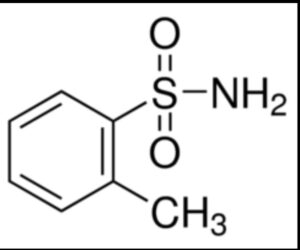
o-Toluene sulfonamide was mainly produced as a chemical intermediate for the production of saccharin. A mixture of o-Toluene sulfonamide with the p-isomer is used as a plasticizer for hot -melt adhesives, a chemical intermediate for fluorescent pigments and a chemical intermediate for plasticizer resins.
P-toluene sulfonamide
Structure –
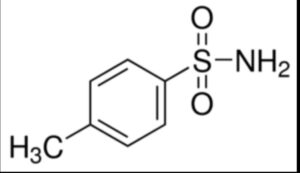
Toluene-4-sulfonamide is a sulfonamide that is benzenesulfonamide bearing a methyl group at position 4.
It is used in the manufacture of dyes, synthetic resins, paints, disinfectants and wood processing brightener.
It is used for the manufacture of plasticizers, disinfectants; it can also be used for the production of synthetic resins, paints, and fluorescent dyes.
Tributyl o-acetyl citrate/Acetyl Tributyl citrate
Structure –
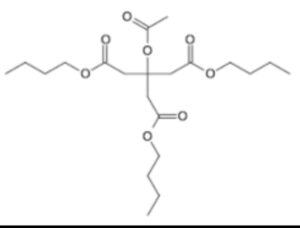
Acetyltributylcitrate is an organic compound that is used as a plasticizer. As such, it is a potential replacement of DEHP and DINP. It is a colorless liquid that is soluble in organic solvents. It is found in nail polish and other cosmetics. It is prepared by acetylation of tributylcitrate
Vildagliptin Impurity D
Structure –
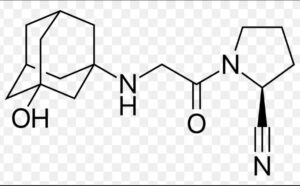
Vildagliptin, sold under the brand name Galvus among others, is an oral anti-hyperglycemic agent (anti-diabetic drug) of the dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of GLP-1 and GIP by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans in the pancreas.
Vildagliptin has been shown to reduce hyperglycemia in type 2 diabetes mellitus
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)


