Summary :-Mirati Therapeutics, Inc. (NASDAQ: MRTX), a targeted oncology company, today announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for KRAZATI™ (adagrasib), a targeted treatment option for adult patients with KRASG12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy.

Mirati Therapeutics Announces U.S. FDA Accelerated Approval of KRAZATI™ (adagrasib) as a Targeted Treatment Option for Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) with a KRASG12C Mutation
KRAZATI has demonstrated a positive benefit-risk profile with accelerated approval based on the Phase 2 registration-enabling cohort of the KRYSTAL-1 study, evaluating KRAZATI 600 mg capsules administered orally twice daily in 116 patients with KRASG12C-mutated advanced NSCLC who previously received treatment with a platinum-based regimen and an immune checkpoint inhibitor. The primary efficacy endpoints were confirmed ORR and DOR as evaluated by blinded independent central review (BICR) according to response evaluation criteria in solid tumors (RECIST v1.1).
The trial demonstrated an ORR of 43% (95% CI: 34-53) with 80% (95% CI: 71-87) of patients achieving disease control. The median DOR was 8.5 months (95% CI: 6.2-13.8).
About KRAZATI (adagrasib)
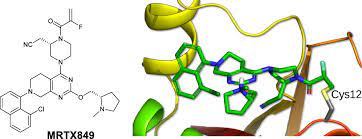
Adagrasib Structure
Mirati has risen to meet one of the most challenging mutations in cancer research by developing KRAZATI, a highly selective and potent oral small-molecule inhibitor of KRASG12C.
Intentionally designed to meet the challenge of KRASG12C, adagrasib is optimized to sustain target inhibition, an attribute that could be important to treat KRASG12C-mutated cancers, as the KRASG12C protein regenerates every 24−48 hours. Adagrasib has shown clinically to be a CNS penetrant, which may be important given that CNS metastases frequently occur in NSCLC and lead to poor prognosis.
In the U.S., KRAZATI was reviewed by the FDA for Accelerated Approval (Subpart H), which allows for the approval of drugs that treat serious conditions, and that fill an unmet medical need based on surrogate endpoints. KRAZATI was reviewed under the FDA Real-Time Oncology Review (RTOR) pilot program, which aims to explore a more efficient review process that ensures safe and effective treatments are made available to patients as early as possible. Mirati submitted a Marketing Authorization Application (MAA) in the EU in May 2022. In 2021, adagrasib achieved Breakthrough Therapy Designation in the U.S. as a potential treatment for patients with NSCLC harboring the KRASG12C mutation who have received at least one prior systemic therapy.
KRAZATI (adagrasib) U.S. Indication
KRAZATI is indicated for the treatment of adult patients with KRASG12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test, who have received at least one prior systemic therapy.
This indication is approved under accelerated approval based on objective response rate (ORR) and duration of response (DOR). Continued approval for this indication may be contingent upon verification and description of a clinical benefit in a confirmatory trial(s).
KRAZATI (adagrasib) Important Safety Information
WARNINGS AND PRECAUTIONS
Gastrointestinal Adverse Reactions
- In the pooled safety population, serious gastrointestinal adverse reactions observed were gastrointestinal obstruction in 1.6%, including 1.4% grade 3 or 4, gastrointestinal bleeding in 0.5% of patients, including 0.5% grade 3, and colitis in 0.3%, including 0.3% grade 3. In addition, nausea, diarrhea, or vomiting occurred in 89% of 366 patients, including 9% grade 3. Nausea, diarrhea, or vomiting led to dosage interruption or dose reduction in 29% of patients and permanent discontinuation of KRAZATI in 0.3%
- Monitor and manage patients using supportive care, including antidiarrheals, antiemetics, or fluid replacement, as indicated. Withhold, reduce the dose, or permanently discontinue KRAZATI based on severity
QTc Interval Prolongation
- KRAZATI can cause QTc interval prolongation, which can increase the risk for ventricular tachyarrhythmias (eg, torsades de pointes) or sudden death
- In the pooled safety population, 6% of 366 patients with at least one post-baseline electrocardiogram (ECG) assessment had an average QTc ≥501 ms, and 11% of patients had an increase from baseline of QTc >60 msec. KRAZATI causes concentration-dependent increases in the QTc interval
- Avoid concomitant use of KRAZATI with other products with a known potential to prolong the QTc interval. Avoid use of KRAZATI in patients with congenital long QT syndrome and in patients with concurrent QTc prolongation
- Monitor ECGs and electrolytes prior to starting KRAZATI, during concomitant use, and as clinically indicated in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, and in patients who are taking medications that are known to prolong the QT interval. Withhold, reduce the dose, or permanently discontinue KRAZATI, depending on severity
Hepatotoxicity
- KRAZATI can cause hepatotoxicity
- In the pooled safety population, hepatotoxicity occurred in 37%, and 7% were grade 3 or 4. A total of 32% of patients who received KRAZATI had increased alanine aminotransferase (ALT)/increased aspartate aminotransferase (AST); 5% were grade 3 and 0.5% were grade 4. Increased ALT/AST leading to dose interruption or reduction occurred in 11% of patients. KRAZATI was discontinued due to increased ALT/AST in 0.5% of patients
- Monitor liver laboratory tests (AST, ALT, alkaline phosphatase, and total bilirubin) prior to the start of KRAZATI, and monthly for 3 months or as clinically indicated, with more frequent testing in patients who develop transaminase elevations. Reduce the dose, withhold, or permanently discontinue KRAZATI based on severity
Interstitial Lung Disease /Pneumonitis
- KRAZATI can cause interstitial lung disease (ILD)/pneumonitis, which can be fatal. In the pooled safety population, ILD/pneumonitis occurred in 4.1% of patients, 1.4% were grade 3 or 4, and 1 case was fatal. The median time to first onset for ILD/pneumonitis was 12 weeks (range: 5 to 31 weeks). KRAZATI was discontinued due to ILD/pneumonitis in 0.8% of patients
- Monitor patients for new or worsening respiratory symptoms indicative of ILD/pneumonitis (eg, dyspnea, cough, fever). Withhold KRAZATI in patients with suspected ILD/pneumonitis and permanently discontinue KRAZATI if no other potential causes of ILD/pneumonitis are identified
Adverse Reactions
- The most common adverse reactions (≥25%) are nausea, diarrhea, vomiting, fatigue, musculoskeletal pain, hepatotoxicity, renal impairment, edema, dyspnea, decreased appetite
Females and Males of Reproductive Potential
- Infertility: Based on findings from animal studies, KRAZATI may impair fertility in females and males of reproductive potential
About the KRYSTAL-1 Study
KRYSTAL-1 is an open-label Phase 1/2 multiple-expansion cohort trial evaluating adagrasib as monotherapy and in combination with other anti-cancer therapies in patients with advanced solid tumors harboring the KRASG12C mutation.
About KRASG12C in NSCLC
Lung cancer is one of the most common cancers worldwide, accounting for 2.21 million new cases and 1.8 million deaths worldwide in 2020. Lung cancer consists of NSCLC in approximately 85% of cases and small cell lung cancer (SCLC) in approximately 15% of cases. KRASG12C is the most common KRAS mutation in NSCLC, present in approximately 14% of patients with lung adenocarcinoma, and is a biomarker mutation of poor prognosis.
About Mirati Therapeutics, Inc.
Mirati Therapeutics, Inc. is a commercial-stage biotechnology company whose mission is to discover, design and deliver breakthrough therapies to transform the lives of patients with cancer and their loved ones. The company is relentlessly focused on bringing forward therapies that address areas of high unmet need, including lung cancer, and advancing a pipeline of novel therapeutics targeting the genetic and immunological drivers of cancer. Unified for patients, Mirati’s vision is to unlock the science behind the promise of a life beyond cancer.
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules.
-
- Weblink: https://chemroboticspharma.com/pharmVetPat

