- The U.S. Food and Drug Administration (FDA) on Friday approved Biogen and Sage Therapeutics oral pill to treat postpartum depression (PPD) in adults.
- The approval of ZURZUVAE to treat women with PPD is based on the NEST clinical development program, which included two studies in adult women with PPD (ROBIN and SKYLARK Studies).
About Zurzuvae
- API– Zuranolone
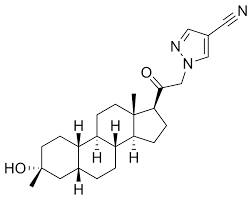
Zuranolone Structure
- Description– Zurzuvae (Zuranolone) is the first pill for treating postpartum depression in adults. Before this, the only option was a IV injection given by a healthcare provider. The most common side effects include drowsiness, dizziness, diarrhea, fatigue, nasopharyngitis, and urinary tract infection. Zuranolone is an oral neuroactive steroid (NAS) GABA-A receptor positive allosteric modulator (PAM).
- IUPAC– 1-[2-[(3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethyl-2,4,5,6,7,8,9,10,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl]pyrazole-4-carbonitrile
- CAS RN– 1632051-40-1
- Originator- SAGE Therapeutics
- Developer- SAGE Therapeutics; Shionogi
- Global Approval:
- USFDA – Aug 05, 2023
- Japan – Completing a Phase 2 trial
- Taiwan – Under clinical trial
- South Korea – Under clinical trial
- Patent Disclosures – WO2014169833 (PCT/CN2014/075594), a Novel compound patent filed by Sage Therapeutics in the year 2014.
- Expiry based on International application – April 17 ,2034
- Product Patent Synthesis and Other Manufacturing Process: Access ChemRobotics PharmVetPat
- Access Other Patents including Compound, Process, Composition, Polymorph and Combination Patents: – Access ChemRobotics PharmVetPat
- Raw Material or intermediate Report: Access ChemRobotics PharmVetPat
- Mechanism of Action-GABA A receptor modulators
- Label Indication – ZURZUVAE is a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator indicated for the treatment of postpartum depression (PPD) in adults.
- Dosage -The daily recommended dose for Zurzuvae is 50mg. It should be taken once every day, for 14 days, in the evening with a fatty meal.
- Collaboration:-
- In June 2018, Sage and Shionogi entered into a strategic collaboration to develop and commercialize zuranolone (SAGE-217) in major depressive disorder (MDD) and other indications in Japan, Taiwan, and South Korea. The goal of the collaboration is to accelerate development of a potential medicine for patients in key Asian markets. Sage’s collaboration with Shionogi continues to advance, with Shionogi recently completing a Phase 2 trial with zuranolone (SAGE-217) in Japan in the treatment of MDD. Under terms of collaboration, Shionogi is responsible for all clinical development, regulatory filings and commercialization of Zuranolone (SAGE-217) in MDD, and potentially other indications, in Japan, Taiwan and South Korea.
- In November 2020, Sage and Biogen Inc. entered into a global collaboration and license agreement to jointly develop and commercialize zuranolone (SAGE-217) in major depressive disorder (MDD) and postpartum depression (PPD), as well as SAGE-324 in essential tremor (ET) and other movement disorders. Sage and Biogen are jointly developing, and, if FDA approved, will jointly commercialize, zuranolone (SAGE-217) and SAGE-324 in the U.S. Biogen has an exclusive license to develop and commercialize zuranolone (SAGE-217) and SAGE-324 outside of the U.S., excluding rights to zuranolone (SAGE-217) in Japan, Taiwan and South Korea.
About Biogen Inc
Biogen Inc. is an American multinational biotechnology company based in Cambridge, Massachusetts, specializing in the discovery, development, and delivery of therapies for the treatment of neurological diseases to patients worldwide.
About Sage Therapeutics
Sage is a biopharmaceutical company committed to developing novel therapies with the potential to transform the lives of people with debilitating disorders of the brain
PharmVetPat® - Pharma || Veterinary || Agrochemicals Active Ingredients and their mixtures with more than 30 physicochemical properties. Users can access the full product landscape report comprising chemistry, including route of synthesis, biology, IP (Patents including Compound Patent / Innovator / Developer Info.) and regulatory info. with product identification including CAS No., developmental code, EPA Codes. Compound and intermediate synthetic routes with raw material including Key Starting Material (KSM) or Intermediate Information. Territory Coverage: USA, Europe, Canada, India, Japan and Korea. View Pharma Database