Summary:-
- INPEFA granted broad label across full range of left ventricular ejection fraction, including HFpEF and HFrEF, and for patients with or without diabetes
- The drug has been rejected before – in 2019, in patients with Type 1 diabetes, on safety grounds.
- The fact that Sanofi was originally Lexicon’s big pharma partner with Sotagliflozin, but pulled out of the partnership after the FDA rejection in 2019 could be telling.
- INPEFA (Sotagliflozin) is a sodium-glucose cotransporter 2 (SGLT2) inhibitor used in the treatment of heart failure.
- European medicines agency (EMA) – Zynquista (Sotagliflozin) – The marketing authorisation for Zynquista has been withdrawn in the EU in August 2022.
The U.S. Food and Drug Administration (FDA) has approved INPEFATM (Sotagliflozin), an oral tablet to be taken once daily, to lower the risk of cardiovascular death, heart failure hospitalisation, and urgent heart failure visit in individuals with:
- Heart failure or
- Type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors.
The broad label encompasses heart failure patients across the full range of left ventricular ejection fraction (LVEF), including preserved ejection fraction and reduced ejection fraction, and for patients with or without diabetes.
About INPEFATM
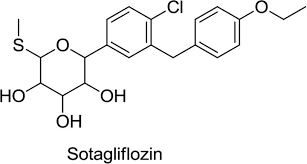
- API Name – Sotagliflozin
- Development Code: LP 802034, LX4211
- CAS RN: 1018899-04-1
- Pubchem ID: 24831714
- Description– Discovered using Lexicon’s unique approach to gene science, INPEFA™ (Sotagliflozin) is an oral inhibitor of two proteins responsible for glucose regulation known as sodium-glucose cotransporter types 2 and 1 (SGLT2 and SGLT1). SGLT2 is responsible for glucose reabsorption by the kidney and SGLT1 is responsible for glucose absorption in the gastrointestinal tract. Sotagliflozin has been studied in multiple patient populations encompassing heart failure, diabetes, and chronic kidney disease in clinical studies involving approximately 20,000 patients.
- Originator (Innovator) – Lexicon Pharmaceuticals
- Developer – Lexicon Pharmaceuticals; Sanofi
- Mechanism of Action – Sodium-glucose transporter 1 inhibitors; Sodium-glucose transporter 2 inhibitors
- Date of Approval- May 26, 2023
- Patent disclosures – WO2008042688 (PCT/US2007/079654), a compound patent filed by Lexicon Pharmaceuticals, Inc. in the year 2007. This disclosure introduces a range of novel SGLT2 inhibitors that hold immense potential for medical advancements. The invention encompasses pharmaceutical compositions, methods of inhibiting SGLT2 activity, as well as methods of treating, preventing, and managing a variety of diseases and disorders. Additionally, WO2019166958 (PCT/IB2019/051559), a process patent related to Sotagliflozin, which may be used to treat diabetes is prepared in this disclosure using schemes and intermediates.
- Expiry based on international application – Sept 27, 2027
- Label Indication: INPEFA is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visit in adults with:
- Heart failure or
- Type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors.
Drug Interactions:
- Digoxin: Monitor patients appropriately as there is an increase in the exposure of digoxin when coadministered with INPEFA 400 mg.
- Uridine 5′-diphospho-glucuronosyltransferase (UGT) Inducer: The coadministration of rifampicin, an inducer of UGTs, with sotagliflozin resulted in a decrease in the exposure of sotagliflozin.
- Lithium: Concomitant use of an SGLT2 inhibitor with lithium may decrease serum lithium concentrations. Monitor serum lithium concentration more frequently during INPEFA initiation and with dosage changes.
About Heart Failure:
About 6.7 million Americans suffer from heart failure, a progressive, debilitating condition that is becoming more prevalent. Heart failure is the leading cause of hospitalizations for individuals aged 65 and older, triggering approximately 1.3 million hospitalizations a year.
About the SOLOIST-WHF Study:
SOLOIST-WHF was a multi-center, randomized, double-blinded, placebo-controlled Phase 3 study evaluating the cardiovascular efficacy of sotagliflozin versus placebo when added to standard of care in 1,222 patients with type 2 diabetes who had recently been hospitalized for worsening heart failure. The primary endpoint was the total number of events comprised of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure in patients treated with sotagliflozin compared with placebo.
SOLOIST-WHF achieved its primary endpoint, with overall tolerability similar to placebo. Results were presented at the Late-Breaking Science Session of the American Heart Association (AHA) Scientific Sessions 2020 and simultaneously published in The New England Journal of Medicine (NEJM) in an article titled: “Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure.”
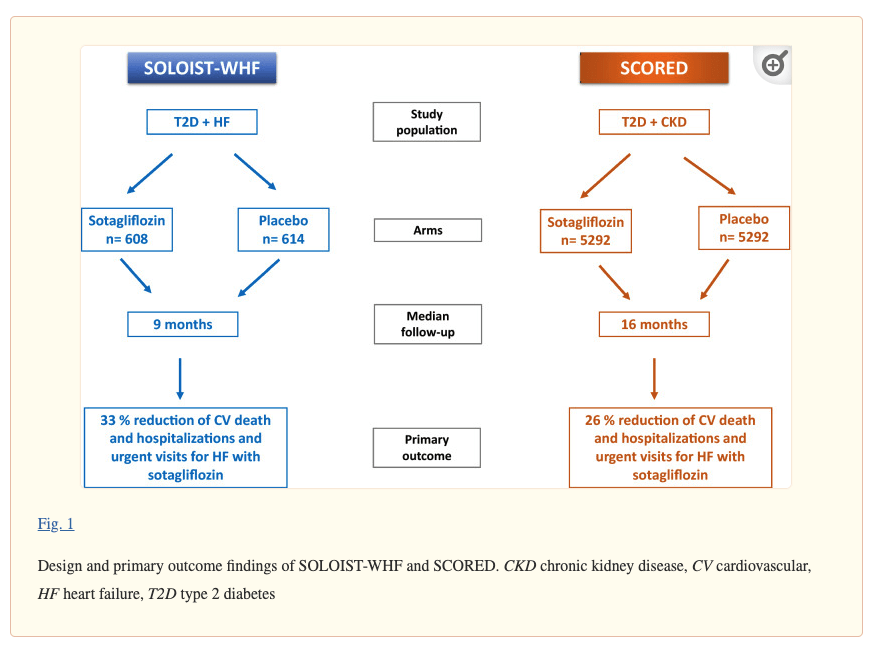
About the SCORED Study
SCORED was a multi-center, randomized, double-blinded, placebo-controlled Phase 3 study evaluating the cardiovascular efficacy of sotagliflozin versus placebo when added to standard of care in 10,584 patients with type 2 diabetes, chronic kidney disease with eGFR of 25 ml to 60 ml per minute per 1.73 m2 of body-surface area, and risks for cardiovascular disease. The primary endpoint was the total number of events comprised of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure in patients treated with sotagliflozin compared with placebo. Key secondary endpoints included total number of events of deaths from cardiovascular causes, non-fatal myocardial infarction, and non-fatal stroke.
SCORED achieved its primary endpoint, with overall tolerability similar to placebo. Results were presented at the Late-Breaking Science Session of the American Heart Association (AHA) Scientific Sessions 2020 and simultaneously published in The New England Journal of Medicine (NEJM) in an article titled: “Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease.”
About Lexicon Pharmaceuticals:
Lexicon is a biopharmaceutical company with a mission of pioneering medicines that transform patients’ lives. Through its Genome5000™ program, Lexicon scientists studied the role and function of nearly 5,000 genes and identified more than 100 protein targets with significant therapeutic potential in a range of diseases. Through the precise targeting of these proteins, Lexicon is pioneering the discovery and development of innovative medicines to treat diseases safely and effectively. Lexicon has previously advanced one of these medicines to market and has a pipeline of promising drug candidates in heart failure, neuropathic pain, diabetes and metabolism and other indications.
PharmVetPat® – Pharma || Veterinary || Agrochemicals
Active Ingredients and their mixtures with more than 30 physicochemical properties. Users can access the full product landscape report comprising chemistry, including route of synthesis, biology, IP (Patents including Compound Patent / Innovator / Developer Info.) and regulatory info. with product identification including CAS No., developmental code, EPA Codes. Compound and intermediate synthetic routes with raw material including Key Starting Material (KSM) or Intermediate Information.
Territory Coverage: USA, Europe, Canada, India, Japan and Korea.
View Pharma Database.