Synopsis:-
- Joenja is the first drug approved in the U.S. for the treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older and is scheduled to launch in early April.
- Under the terms of Pharming’s 2019 exclusive license agreement with Novartis for leniolisib, the corresponding first commercial sale of Joenja® triggers a $10 million milestone payment by Pharming to Novartis.
About Joenja®
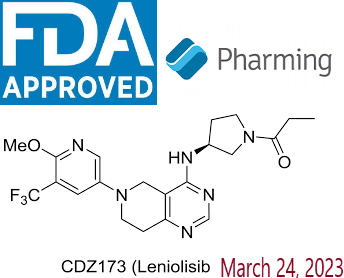
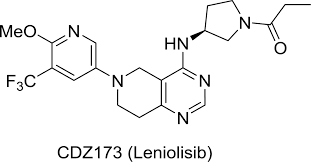
- Active Ingredient – Leniolisib
 Description-Joenja® (leniolisib) is an oral small molecule phosphoinositide 3-kinase delta (PI3Kẟ) inhibitor approved in the US as the first and only targeted treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older. Joenja® inhibits the production of phosphatidylinositol-3-4-5-trisphosphate, which serves as an important cellular messenger and regulates a multitude of cell functions such as proliferation, differentiation, cytokine production, cell survival, angiogenesis, and metabolism.
Description-Joenja® (leniolisib) is an oral small molecule phosphoinositide 3-kinase delta (PI3Kẟ) inhibitor approved in the US as the first and only targeted treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older. Joenja® inhibits the production of phosphatidylinositol-3-4-5-trisphosphate, which serves as an important cellular messenger and regulates a multitude of cell functions such as proliferation, differentiation, cytokine production, cell survival, angiogenesis, and metabolism.- IUPAC – 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one
- CAS RN – 1354690-24-6
- Originator – Novartis
- Developer – Novartis , Pharming Group N.V.
- FDA Approved : First approved March 24, 2023
- Patent Disclosures – WO2012004299 (PCT/EP2011/0613933), a Novel compound patent filed by Novartis AG in the year 2011. This patent discloses about the substituted tetrahydro-pyrido-pyridimidine derivatives which are useful for the treatment of a disorder or disease which is mediated by the activity of the PI3K enzymes and the patent further discloses the process of preparation of the same.
- USFDA – NCE Exclusivity: The compound got NCE exclusivity till 03/24/2028
- USFDA – ODE Exclusivity: The compound got ODE-430 exclusivity till 03/24/2030
- Product Patent Synthesis and Other Manufacturing Process: Access ChemRobotics PharmVetPat
- Access Other Patents including Compound, Process, Composition, Polymorph and Combination Patents: – Access ChemRobotics PharmVetPat
- Raw Material or intermediate Report: Access ChemRobotics PharmVetPat
- Expiry based on International application – July 6 ,2031
- Label Indication –JOENJA is a kinase inhibitor indicated for the treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older.
- Mechanism of Action – Phosphatidylinositol 3 kinase delta inhibitors
- Dosage Form– Tablets: 70 mg Leniolisib
- Designations -Joenja received orphan drug designation, rare pediatric disease designation, and priority review.
- Commercialization – After Joenja hits the market in April, Novartis and the other party will also be eligible for up to $190 million in sales milestones and tiered royalties of low double-digit to a high-teen double-digit percentage of net sales and in April 11, 2023 -Pharming Group N.V. announces the first commercial shipments of Joenja® (leniolisib) to patients in the United States.
About Activated Phosphoinositide 3-Kinase δ Syndrome (APDS)
APDS is a rare primary immunodeficiency that was first characterized in 2013. APDS is caused by variants in either one of two identified genes known as PIK3CD or PIK3R1, which are vital to the development and function of immune cells in the body. Variants of these genes lead to hyperactivity of the PI3Kδ (phosphoinositide 3-kinase delta) pathway, which causes immune cells to fail to mature and function properly, leading to immunodeficiency and dysregulation.
About Pharming Group N.V.
Pharming Group N.V. is a global biopharmaceutical company dedicated to transforming the lives of patients with rare, debilitating, and life-threatening diseases. Pharming is commercializing and developing an innovative portfolio of protein replacement therapies and precision medicines, including small molecules, biologics, and gene therapies that are in early to late-stage development.
About NOVARTIS
Novartis AG is a Swiss multinational pharmaceutical corporation in Basel, Switzerland . Globally ranked in top 5 , is one of the largest pharmaceutical companies . in terms of revenue ,its fourth largest in 2022. They provide solutions addressing the needs of the patients worldwide.
PharmVetPat® - Pharma || Veterinary || Agrochemicals Active Ingredients and their mixtures with more than 30 physicochemical properties. Users can access the full product landscape report comprising chemistry, including route of synthesis, biology, IP (Patents including Compound Patent / Innovator / Developer Info.) and regulatory info. with product identification including CAS No., developmental code, EPA Codes. Compound and intermediate synthetic routes with raw material including Key Starting Material (KSM) or Intermediate Information. Territory Coverage: USA, Europe, Canada, India, Japan and Korea. View Pharma Database


 Description-Joenja® (leniolisib) is an oral small molecule phosphoinositide 3-kinase delta (PI3Kẟ) inhibitor approved in the US as the first and only targeted treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older. Joenja® inhibits the production of phosphatidylinositol-3-4-5-trisphosphate, which serves as an important cellular messenger and regulates a multitude of cell functions such as proliferation, differentiation, cytokine production, cell survival, angiogenesis, and metabolism.
Description-Joenja® (leniolisib) is an oral small molecule phosphoinositide 3-kinase delta (PI3Kẟ) inhibitor approved in the US as the first and only targeted treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in adult and pediatric patients 12 years of age and older. Joenja® inhibits the production of phosphatidylinositol-3-4-5-trisphosphate, which serves as an important cellular messenger and regulates a multitude of cell functions such as proliferation, differentiation, cytokine production, cell survival, angiogenesis, and metabolism.