Synopsis
- First and only approved therapy for Rett syndrome, a rare, neurodevelopmental disorder, which affects 6,000 to 9,000 patients in the U.S.
- Trofinetide (DAYBUE™), an oral, small molecule, synthetic analog of glycine-proline-glutamate [GPE; the N-terminal tripeptide derivative of insulin like growth factor-1 (IGF-1)], is being developed by Neuren Pharmaceuticals and Acadia Pharmaceuticals for the treatment of rare childhood neurodevelopmental disorders.
- Trofinetide has been granted Fast Track Status and Orphan Drug Designation in the U.S. and Orphan Drug Designation in Europe for both Rett syndrome and Fragile X syndrome.
- In 2018, Acadia entered into an exclusive license agreement with Neuren Pharmaceuticals Limited for the development and commercialization of Trofinetide for the treatment of Rett syndrome and other indications in North America.
The FDA has approved DAYBUE™ (Trofinetide) for the treatment of Rett syndrome in adult and paediatric patients two years of age and older, according to Acadia Pharmaceuticals Inc.. The first and only medication authorised for the treatment of Rett syndrome is called DAYBUE.
About DAYBUE™
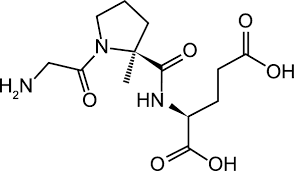
Trofinetide Structure
- API -: Trofinetide
- Description-: Trofinetide (NNZ 2566), a proprietary small molecule analogue of glycine-proline-glutamate [Glypromate®], is being developed by Neuren Pharmaceuticals for the treatment of brain injuries, Fragile X syndrome, Rett syndrome. Trofinetide is a synthetic analogue of a naturally occurring neurotrophic peptide derived from IGF-1, a growth factor produced by brain cells. In animal models, trofinetide exhibits a wide range of important effects including inhibiting neuroinflammation, normalizing the role of microglia and correcting deficits in synaptic function. Trofinetide is being developed both in intravenous and oral formulations for a range of acute and chronic conditions. It has been granted Fast Track Status and Orphan Drug Designation in the U.S. and Orphan Drug Designation in Europe for both Rett syndrome and Fragile X syndrome.
- IUPAC-: (2S)-2-[[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino]pentanedioic acid
- CAS RN-: 853400-76-7
- Originator-: Neuren Pharmaceuticals
- Developer-: ACADIA Pharmaceuticals; Neuren Pharmaceuticals; Walter Reed Army Institute of Research
- FDA Approval-: First approved March 10, 2023
- USFDA – NCE Exclusivity: The compound got NCE exclusivity till 03/10/2028
- USFDA – ODE Exclusivity: The compound got ODE-425 exclusivity till 03/10/2030
- Patent disclosures :- WO2002094856 (PCT/US2002/016361) Patent filled by the originator company Neuren Pharmaceuticals in the year 2002. The disclosure of patent relates to the analogs and peptidomimetics of glycyl-L-prolyl-L-glutamic acid (GPE) , specifically coordinated with GPE analogs and peptidomimetics that are anti-apoptotic and anti-necrotic.
- In addition, the patent with the number US11370755 reveals details about pharmaceutical compositions containing Trofinetide. Similarly, the patent registered as US9212204 provides information regarding a method for treating Rett Syndrome.
- Polymorphism: Trofinetide and trofinetide hydrate, pharmaceutical compositions is disclosed in US20230023114.
- Product Patent Synthesis and Other Manufacturing Process: Access ChemRobotics PharmVetPat
- Access Other Patents including Compound, Process, Composition, Polymorph and Combination Patents: – Access ChemRobotics PharmVetPat
- Raw Material or intermediate Report: Access ChemRobotics PharmVetPat
- Label Indication-: DAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
- Mechanism of Action-: Astrocyte inhibitors; Cytokine inhibitors; Glial cell inhibitors; Insulin-like growth factor I stimulants
- Orphan Drug Status-: Yes – Fragile X syndrome; Rett syndrome
- Dosage form-: Oral Solution
- Drug Interactions: Effect of DAYBUE on other Drugs
- DAYBUE is a weak CYP3A4 inhibitor; therefore, plasma concentrations of CYP3A4 substrates may be increased if given concomitantly with DAYBUE. Closely monitor when DAYBUE is used in combination with orally administered CYP3A4 sensitive substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
- Plasma concentrations of OATP1B1 and OATP1B3 substrates may be increased if given concomitantly with DAYBUE. Avoid the concomitant use of DAYBUE with OATP1B1 and OATP1B3 substrates for which a small change in substrate plasma concentration may lead to serious toxicities.
About Rett Syndrome
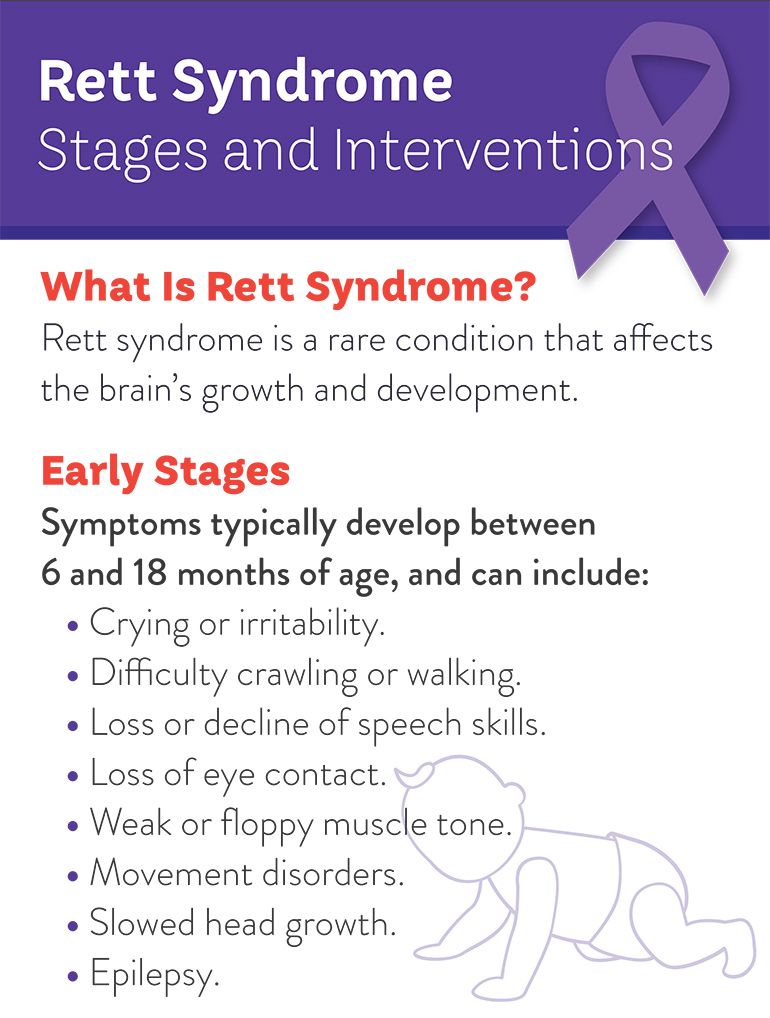
Rett syndrome is a rare, complex, neurodevelopmental disorder that may occur over four stages and affects approximately 6,000 to 9,000 patients in the U.S., with approximately 4,500 patients currently diagnosed according to an analysis of healthcare claims data. In preclinical studies, deficiency in MeCP2 function has been shown to lead to impairment in synaptic communication, and the deficits in synaptic function may be associated with Rett manifestations. Rett is characterized by progressive decline in motor, cognitive and communicative functioning starting when child is between 5-18 months old.
The decline follows four stages:
Stage 1: Stagnation
Stage 2: Regression
Stage 3: Stationary
Stage 4: Motor deterioration
About Neuren Pharmaceuticals
Neuren Pharmaceuticals Limited is an Australia-based biopharmaceutical company that is engaged in developing drugs for neurological disorders. The company is developing new therapies for six debilitating neurodevelopmental disorders that emerge in early childhood and are characterised by impaired connections and signalling between brain cells.
About Acadia Pharmaceuticals
Acadia is advancing breakthroughs in neuroscience to elevate life. For almost 30 years we have been working at the forefront of healthcare to bring vital solutions to people who need them most. The company developed and commercialized the first and only approved therapies for hallucinations and delusions associated with Parkinson’s disease psychosis and for the treatment of Rett syndrome.
PharmVetPat® – Pharma || Veterinary || Agrochemicals
Active Ingredients and their mixtures with more than 30 physicochemical properties. Users can access the full product landscape report comprising chemistry, including route of synthesis, biology, IP (Patents including Compound Patent / Innovator / Developer Info.) and regulatory info. with product identification including CAS No., developmental code, EPA Codes. Compound and intermediate synthetic routes with raw material including Key Starting Material (KSM) or Intermediate Information.
Territory Coverage: USA, Europe, Canada, India, Japan and Korea.
View Pharma Database