- Novartis says that the U.S. and European regulators have accepted its marketing applications for CAR-T cell therapy Kymriah (Tisagenlecleucel) as third-line therapy for adults with relapsed or refractory (r/r) follicular lymphoma (FL).
- Kymriah is already approved in the U.S. and Europe for acute lymphoblastic leukemia and diffuse large B-cell lymphoma (DLBCL).
- The FDA has accepted the company’s Supplemental Biologics License Application (sBLA) for Kymriah with priority review, while the European Medicines Agency (EMA) has accepted the Type II Variation for the treatment.
- Kymriah already has an orphan medicinal product designation from the European Commission (EC) for FL.
- Filings supported by pivotal ELARA trial, where treatment with Kymriah showed robust response rates and remarkable safety profile in adult patients with relapsed or refractory (r/r) follicular lymphoma (FL).
- Kymriah also received orphan drug designation from the European Commission (EC) for patients with FL earlier this year.
- Patients with FL who are refractory to treatment or relapse after two prior lines are in need of durable alternatives to traditional therapies as the efficacy of treatments decreases in later lines2.
- Kymriah, the first-ever FDA-approved CAR-T cell therapy, is currently available in 30 countries in one or more indications, with more than 345 certified treatment centers worldwide.

Novartis announced that the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have accepted the company’s Supplemental Biologics License Application (sBLA).
Novartis announced that the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have accepted the company’s Supplemental Biologics License Application (sBLA) and Type II Variation, respectively, for Kymriah (Tisagenlecleucel) in adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two prior lines of treatment.
The FDA has also granted priority review to the company’s sBLA for Kymriah in adult patients with r/r FL. Kymriah was previously granted orphan medicinal product designation by the European Commission (EC) for FL. If approved in this potential third indication, Kymriah would have the opportunity to present an important treatment option for those patients with r/r FL in need of potentially definitive outcomes.
The regulatory submissions are based on positive data from the pivotal phase II ELARA trial, which investigated the efficacy and safety of Kymriah in adult patients with r/r FL. The trial met the primary endpoint with robust responses observed in heavily pretreated patients. The safety profile was remarkable, with no patients experiencing grade 3 or higher cytokine release syndrome (CRS) related to Kymriah within the first 8 weeks following infusion1. Data from the trial was presented earlier this year as an oral presentation during the 2021 Annual American Society of Clinical Oncology (ASCO) Virtual Scientific Meeting.
“This is an important milestone in our mission to bring Kymriah to adult patients with relapsed or refractory follicular lymphoma. Receiving orphan drug designation from the EC as well as priority review from the FDA underscores the unmet need and urgency for these patients. With Kymriah demonstrating impressive results in the ELARA trial, we are hopeful that we can offer a unique and potentially definitive treatment that minimizes the burden,” said Jeff Legos, executive vice president, global head of oncology & hematology development, Novartis.
Orphan drug designation is reserved for medicines that treat, prevent or diagnose a life-threatening or chronically debilitating rare disease with a prevalence in the EU of below 5 in 10,000 and with either no currently approved method of diagnosis, prevention or treatment or with significant benefit to those affected by the disease. The decision follows a positive opinion from the Committee for Orphan Medicinal Products (COMP) of the EMA. Kymriah also has Orphan Drug designation from the FDA and the Japan Ministry of Health, Labour and Welfare (MHLW) for this disease.
Priority Review is granted to therapies that have the potential to provide significant improvements in the treatment, diagnosis or prevention of serious conditions, as determined by the FDA.
Kymriah is currently approved by the FDA, EMA and other regulatory authorities for the treatment of r/r pediatric and young adult (up to and including 25 years of age) acute lymphoblastic leukemia (ALL), and r/r adult diffuse large B-cell lymphoma (DLBCL).
What is follicular lymphoma?
Follicular lymphoma (FL), the second most common form of non-Hodgkin lymphoma (NHL), is an indolent lymphoma, and represents approximately 22% of NHL cases. It is often an unrelenting malignancy with a relapsing and remitting pattern. Throughout the lifetime of a patient with relapsing FL, he or she may be exposed to a median of five lines of prior treatment, with an upper range of 13 lines. Although patients in in third or later line treatment for FL have multiple systemic therapies available, the efficacy of these regimens drops off rapidly in later lines2. Additionally, because of this relapsing and remitting pattern, patients who are refractory to treatment or relapse may exhaust available treatment options.
What are the ELARA trials?
ELARA is a Phase II, single-arm, multicenter, open-label trial investigating the efficacy and safety of Kymriah in adult patients with r/r FL after at least two prior therapies. This international trial has enrolled patients from over 30 sites in 12 countries worldwide. The primary endpoint is complete response rate (CRR) based on best response by central review (Lugano 2014 criteria). Patients evaluable for efficacy had measurable disease at infusion and more than six months of follow-up from infusion or discontinued early. After infusion, disease assessments were performed every three months. Secondary endpoints include overall response rate, duration of response, progression-free survival, overall survival and safety. Primary analysis data announced at ASCO 2021 showed Kymriah led to responses for the majority of patients treated, with 66% achieving a complete response (95% CI, 56-75). The overall response rate was 86% (95% CI, 78-92)1. Importantly, no patients in ELARA trial experienced grade 3 or higher cytokine release syndrome related to Kymriah within the first 8 weeks following infusion, the most common side effect associated with CAR-T therapy1.
About Novartis Commitment to Oncology Cell & Gene:
Novartis has a mission to reimagine medicine by bringing curative cell & gene therapies to patients worldwide. Novartis has a deep CAR-T pipeline and ongoing investment in manufacturing and supply chain process improvements. With active research underway to broaden the impact of cell and gene therapy in oncology, Novartis is going deeper in hematological malignancies, reaching patients with other cancer types and evaluating next-generation CAR-T cell therapies that focus on new targets and utilize new technologies.
Novartis was the first pharmaceutical company to significantly invest in pioneering CAR-T research and initiate global CAR-T trials. Kymriah, the first approved CAR-T cell therapy, developed in collaboration with the Perelman School of Medicine at the University of Pennsylvania, is the foundation of Novartis’ commitment to CAR-T cell therapy.
Kymriah is currently approved for use in at least one indication in 30 countries and at more than 345 certified treatment centers, with the ambition for further expansion to help fulfill the ultimate goal of bringing CAR-T cell therapy to every patient in need.
Novartis has an expansive global CAR-T manufacturing footprint that includes both Novartis-owned and contract manufacturing sites. This comprehensive, integrated footprint strengthens the flexibility, resilience and sustainability of the Novartis manufacturing and supply chain.
What is Kymriah (Tisagenlecleucel)?
Tisagenlecleucel, sold under the brand name Kymriah, is a medication for the treatment of B-cell acute lymphoblastic leukemia (ALL) which uses the body’s own T cells to fight cancer (adoptive cell transfer).
A drug used to treat adults with certain types of B-cell non-Hodgkin lymphoma and people up to 25 years old with certain types of B-cell acute lymphoblastic leukemia. It is also being studied in the treatment of other types of cancer. Tisagenlecleucel is made using a patient’s T cells (a type of immune system cell). A gene for a special receptor called chimeric antigen receptor (CAR) is added to the T cells in the laboratory. These changed T cells called CAR T cells are grown in large numbers in the laboratory and given to the patient by infusion. Tisagenlecleucel binds to a protein called CD19, which is found on some leukemia and lymphoma cells. This helps the body’s immune system kill cancer cells. Tisagenlecleucel is a type of CAR T-cell therapy.
Detail about CAR T-cell therapy:
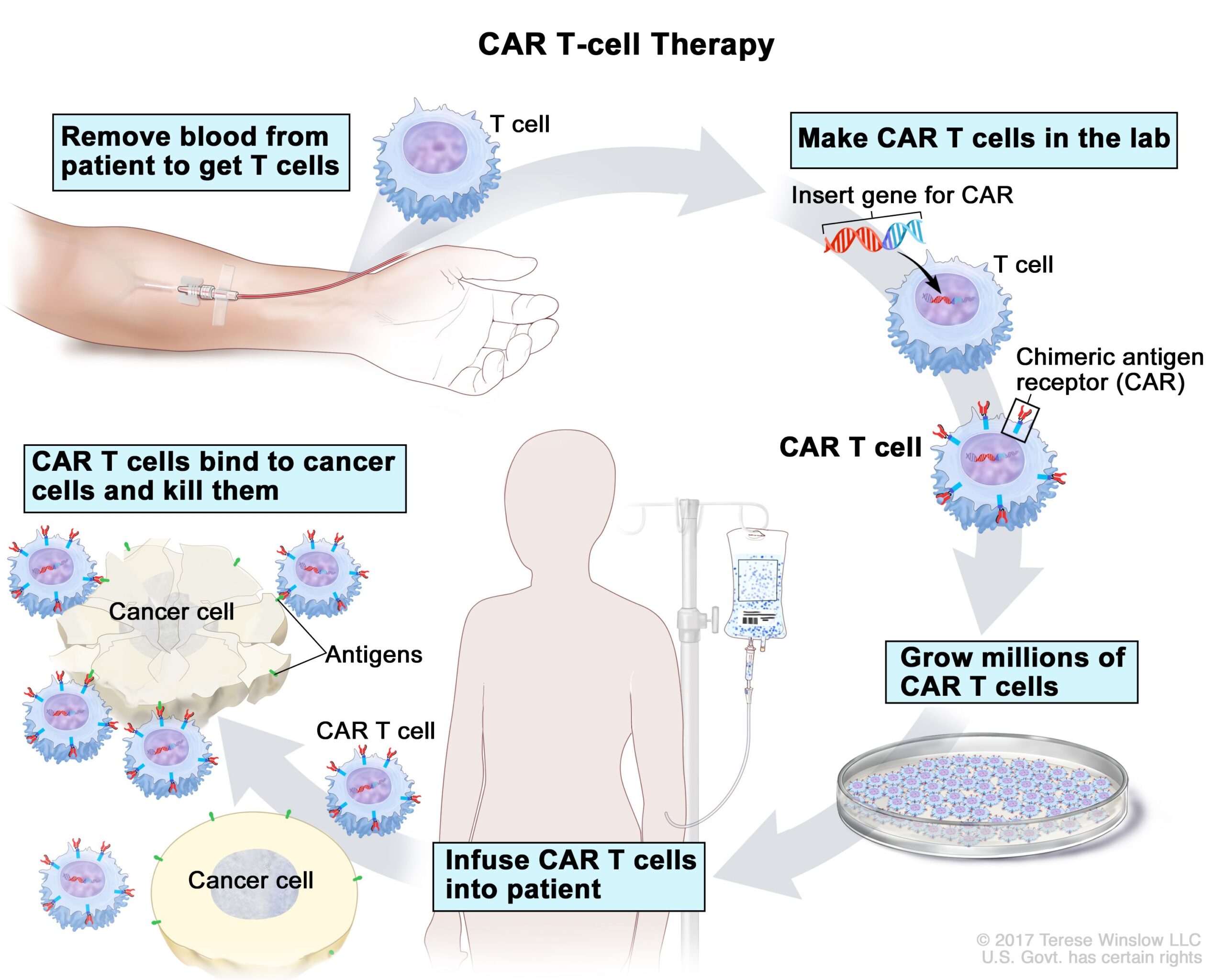
CAR_T cell therapy.
CAR T-cell therapy. A type of treatment in which a patient’s T cells (a type of immune cell) are changed in the laboratory so they will bind to cancer cells and kill them. Blood from a vein in the patient’s arm flows through a tube to an apheresis machine (not shown), which removes the white blood cells, including the T cells, and sends the rest of the blood back to the patient. Then, the gene for a special receptor called a chimeric antigen receptor (CAR) is inserted into the T cells in the laboratory. Millions of the CAR T cells are grown in the laboratory and then given to the patient by infusion. The CAR T cells are able to bind to an antigen on the cancer cells and kill them.
Important Safety Information from the Kymriah SmPC:
Kymriah (Tisagenlecleucel) is an autologous, immunocellular cancer therapy that involves reprogramming a patient’s own T-cells with a transgene encoding a chimeric antigen receptor (CAR) to identify and eliminate CD19-expressing cells. It is administered as intravenous infusion.
Kymriah is indicated for the treatment of pediatric and young adult patients up to and including 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant or in second or later relapse as well as for adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy.
Kymriah must not be administered in case of hypersensitivity to the active substance or to any of the excipients of the product. In addition, contraindications of the lymphodepleting chemotherapy that is usually preceding the Kymriah infusion to prepare the patient’s body, must be considered.
For details, please see the Summary of Product Characteristics (SmPC).
Reasons to delay Kymriah treatment
Kymriah treatment should be delayed, if a patient has any of the following conditions:
- Unresolved serious adverse reactions (especially pulmonary reactions, cardiac reactions or hypotension) from preceding chemotherapies.
- Active uncontrolled infection.
- Active graft-versus-host disease (GVHD).
- Significant clinical worsening of leukemia burden or lymphoma following lymphodepleting chemotherapy.
Important side effects
Kymriah may cause side effects that could be severe, life-threatening or fatal. They usually happen in the first eight weeks after the infusion, but can also develop later. The following main side effects can occur after Kymriah infusion:
- Cytokine release syndromehas been frequently observed and almost always occurred within the first 10 days after Kymriah infusion. Patients may experience high fever, chills, difficulty breathing, nausea, vomiting, diarrhea, muscle pain, joint pain, low blood pressure, dizziness/light headedness, and issues with blood coagulation. Adverse reactions of multiple body organs, such as the heart, the liver or kidney, may occur.
- Neurological events, in particular encephalopathy, confusional state or delirium, can occur frequently with Kymriah. Other manifestations can also include altered or decreased consciousness, agitation, seizures, difficulty speaking, understanding speech, or loss of balance. The majority of neurological events occurred within eight weeks following Kymriah infusion and were transient. Because of the risk of neurological side effects, patients should not drive, operate heavy machinery, or do other activities that require alertness for eight weeks after receiving Kymriah.
- Infections can occur frequently after Kymriah infusion. As appropriate, prophylactic antibiotics should be administered and surveillance testing should be employed prior to and during treatment with Kymriah. Infections are known to complicate the course and management of concurrent cytokine release syndrome. Vaccination with live virus vaccines is not recommended at least six weeks prior to the start of lymphodepleting chemotherapy, during Kymriah treatment, and until immune recovery following treatment with Kymriah.
- Febrile neutropenia was frequently observed in patients after Kymriah infusion. In the event of febrile neutropenia, infection should be evaluated and managed appropriately with broad-spectrum antibiotics, fluids and other supportive care, as medically indicated.
- Tumor lysis syndrome is a rapid breakdown of tumor cells and release of their contents into the bloodstream. This can interfere with the workings of various body organs, especially the kidneys, heart and nervous system. To minimize risk of tumor lysis syndrome, patients with elevated uric acid or high tumor burden should receive allopurinol, or an alternative prophylaxis, prior to Kymriah infusion.
- Prolonged cytopenias, which is a low count of one or more types of blood cells such as red blood cells, white blood cells, or platelets, can persist for several weeks following Kymriah. The majority of patients who had cytopenias at day 28 following Kymriah treatment improved or resolved within three months after treatment. Prolonged neutropenia has been associated with increased risk of infection.
- Hypogammaglobulinemia or Agammaglobulinemia, a condition in which the level of immunoglobulins (antibodies) in the blood is low and the risk of infections is increased, can occur in patients treated with Kymriah. Infection precautions, antibiotic prophylaxis and immunoglobulin replacement should be managed per age and standard guidelines.
- Secondary malignancies: After treatment with Kymriah, patients will be monitored life-long by their healthcare provider, as they may develop secondary cancers.
- Pregnancy and breast-feeding:It is not known, whether Kymriah has the potential to be transferred to the fetus via the placenta and could cause fetal toxicity, including B-cell lymphocytopenia. Kymriah is not recommended during pregnancy and in women of childbearing potential not using contraception. It is unknown, whether Kymriah is excreted in human milk. A risk to the breast-fed infant cannot be excluded. Women, who are breast-feeding, should be advised of the potential risk to the breast-fed infant.
- Blood, organ, tissue and cell donation: Patients treated with Kymriah should not donate blood, organs, tissues and cells for transplantation.
About Novartis
Novartis is reimagining medicine to improve and extend people’s lives. As a leading global medicines company, we use innovative science and digital technologies to create transformative treatments in areas of great medical need. In our quest to find new medicines, we consistently rank among the world’s top companies investing in research and development. Novartis products reach nearly 800 million people globally and we are finding innovative ways to expand access to our latest treatments. About 108,000 people of more than 140 nationalities work at Novartis around the world.



