Summary:
-Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company, today confirms the previously communicated timelines of its clinical trials and regulatory submissions for its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001.
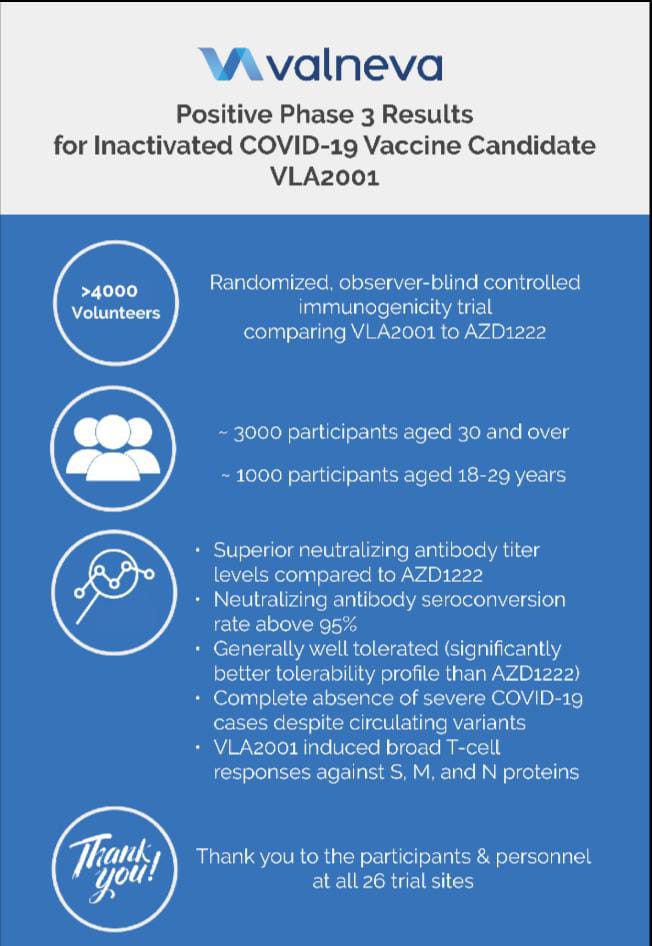
As announced in December 20211, the Company commenced rolling submissions for initial approval of VLA2001 with the European Medicines Agency, the UK MHRA and the Bahraini NHRA, and is continuing to work closely with those authorities to complete their review process following its positive Phase 3 trial results2. Valneva continues to expect potential regulatory approvals in the first quarter of 20221.
The Company also announced positive homologous booster results at the end of December 20213. The data showed an excellent immune response after a third dose of VLA2001 administered seven to eight months after the second dose of primary vaccination. Valneva is also evaluating the sera from the boosted participants for cross-neutralization against Variants of Concern, including Omicron. In parallel, the Company is preparing to launch a dedicated heterologous booster trial, which will evaluate a VLA2001 booster shot provided at least six months after primary vaccination with licensed COVID-19 vaccines or following natural COVID-19 infection.
Valneva , Clinical Trial , Regulatory Submission ,Timelines , COVID-19 ,Vaccine , Candidate , VLA2001 , Phase , Study , Disorder, Pharmanews , Biopharmaceutical, Company
VLA2001 is also being evaluated in elderly and adolescent volunteers. The Company expects to report topline data for the elderly trial in the coming weeks.
Thomas Lingelbach, Chief Executive Officer of Valneva, commented, “In recent weeks, we have been receiving even more messages every day from people around the world who would like to be vaccinated with an inactivated vaccine and want to know more about VLA2001. We continue to believe that our inactivated vaccine candidate could be an important component of the fight against COVID-19, and Valneva remains fully committed to bringing VLA2001 to people who need it as soon as we can. We look forward to sharing further data in due course.”
Valneva announced in November 2021 that the European Commission signed an agreement for the Company to supply up to 60 million doses of VLA2001 over two years – including 24.3 million doses in 20224. Delivery of the vaccine is currently expected to begin in April 2022, subject to approval by the EMA.
About VLA2001
VLA2001 is currently the only whole virus, inactivated, adjuvanted vaccine candidate in clinical trials against COVID-19 in Europe. It is intended for active immunization of at-risk populations to prevent carriage and symptomatic infection with COVID-19 during the ongoing pandemic and potentially later for routine vaccination including addressing new variants. VLA2001 may also be suited for boosting, as repeat booster vaccinations have been shown to work well with whole virus inactivated vaccines. VLA2001 is produced on Valneva’s established Vero-cell platform, leveraging the manufacturing technology for Valneva’s licensed Japanese encephalitis vaccine, IXIARO®. VLA2001 consists of inactivated whole virus particles of SARS-CoV-2 with high S-protein density, in combination with two adjuvants, alum and CpG 1018. This adjuvant combination has consistently induced higher antibody levels in preclinical experiments than alum-only formulations and shown a shift of the immune response towards Th1. CpG 1018 adjuvant, supplied by Dynavax Technologies Corporation (Nasdaq: DVAX), is a component of the US FDA- and EMA-approved HEPLISAV-B® vaccine. VLA2001’s manufacturing process, which has already been upscaled to final industrial scale, includes chemical inactivation to preserve the native structure of the S-protein. VLA2001 is expected to conform with standard cold chain requirements (2 to 8 degrees Celsius).
About Valneva SE
Valneva is a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need. The Company takes a highly specialized and targeted approach to vaccine development and then applies its deep understanding of vaccine science to develop prophylactic vaccines addressing these diseases. Valneva has leveraged its expertise and capabilities both to successfully commercialize two vaccines and to rapidly advance a broad range of vaccine candidates into and through the clinic, including candidates against Lyme disease, the chikungunya virus and COVID-19.
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)

