Summary:
- Xofluza is the first and only single-dose oral medicine for the treatment of influenza to be approved for children as young as five years of age
- The FDA also approved Xofluza to prevent influenza in children aged five and older following contact with an infected person
Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), announced today that the U.S. The Food and Drug Administration (FDA) submitted a supplemental marketing authorization application (sNDA) for Xofluza (baloxavir marboxil) for the treatment of acute uncomplicated influenza in otherwise healthy children ages five to less than 12 years who have been symptomatic for 48 hours or less. This is the first single-dose oral influenza drug approved for children in this age group. In addition, the FDA has approved Xofluza for the prevention (post-exposure prophylaxis) of influenza in children ages five to less than 12 years after exposure to a person with influenza.
About Xofluza
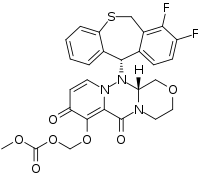
Baloxavir marboxil Structure
- API – Baloxavir marboxil
- Description – Baloxavir marboxil, sold under the brand name Xofluza, is an antiviral medication for treatment of influenza A and influenza B flu. It was approved for medical use both in Japan and in the United States in 2018 and is taken as a single dose by mouth. It may reduce the duration of flu symptoms by about a day, but is prone to selection of resistant mutants that render it ineffectual.
- Developer – Roche; Shionogi
- Indication– Influenza A virus infections; Influenza B virus infections; Influenza virus infections.
- Contraindications –Baloxavir marboxil should not be co-administered with dairy products, calcium-fortified beverages, or laxatives, antacids, or oral supplements containing calcium, iron, magnesium, selenium, aluminum or zinc.
- Side effects –Common side effects following the single dose administration of baloxavir marboxil include diarrhea, bronchitis, common cold, headache, and nausea. Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of oseltamivir.
- Mechanism of Action – Endonuclease inhibitors
Xofluza U.S. Indication
XOFLUZA is a prescription medicine used to:
- treat the flu (influenza) in people who have flu symptoms for no more than 48 hours and who are:
- otherwise healthy adults and children 5 years of age and older, or
- adults and children 12 years of age and older who are at high risk of developing problems from the flu.
- prevent the flu in people 5 years of age and older following contact with a person who has the flu (post-exposure prophylaxis).
Important Safety Information
Who should not take XOFLUZA?
- Do not take XOFLUZA if you are allergic to baloxavir marboxil or any of the ingredients in XOFLUZA.
What should I tell my healthcare provider before using XOFLUZA?
- Tell your healthcare provider about all of your medical conditions, including if you are:
- Pregnant or plan to become pregnant. It is not known if XOFLUZA can harm your unborn baby.
- Breastfeeding or plan to breastfeed. It is not known if XOFLUZA passes into your breast milk.
- Talk to your healthcare provider before you receive a live flu vaccine after taking XOFLUZA.
- Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, antacids, laxatives, vitamins, and herbal supplements.
What are the possible side effects of XOFLUZA?
Serious side effects may include
- Allergic reaction. Get emergency medical help right away if you develop any of the following signs or symptoms of an allergic reaction:
- trouble breathing
- skin rash, hives or blisters
- swelling of your face, throat or mouth
- dizziness or lightheadedness
The most common side effects of XOFLUZA for treatment of the flu in adults and adolescents (12 years of age and older) were diarrhea, bronchitis, nausea, sinusitis, and headache.
The most common side effects of XOFLUZA for treatment of the flu in children (5 years of age to less than 12 years of age) were diarrhea and vomiting.
These are not all the possible side effects of XOFLUZA. Call your healthcare provider for medical advice about side effects.
XOFLUZA is not effective in treating or preventing infections other than influenza. Other kinds of infections can have symptoms like those of the flu or occur along with flu and may need different kinds of treatment. Tell your healthcare provider if you feel worse or develop new symptoms during or after treatment with XOFLUZA or if your flu symptoms do not start to get better.
“Despite the ongoing COVID-19 pandemic, influenza continues to be a threat to public health, and effective influenza antivirals remain critical to alleviating the burden on healthcare systems,” said Levi Garraway, M.D., Ph.D., chief medical officer and head of Global Product Development. “Xofluza has proven to be an important tool in fighting and preventing influenza in adults as well as adolescents, and we are pleased to now offer households and younger children our single-dose oral treatment.”
According to the Centers for Disease Control and Prevention, influenza can be a serious illness for young children. During the ongoing COVID-19 pandemic, there have been significantly fewer influenza cases likely due in large part to social distancing and mask wearing. However, in the U.S. 2018-2019 influenza season, there were more than 6 million illnesses, thousands of hospitalizations and more than 100 deaths for children aged five to 17 caused by influenza.
“Historically, school-aged children have played a significant role in the community transmission of influenza. The annual influenza vaccine continues to be the most important first step to prevent illness in children, though there can still be breakthrough cases where antiviral treatment is needed,” said Dr. Pedro Piedra, miniSTONE-2 study investigator and professor of molecular virology and microbiology, pediatrics at Baylor College of Medicine. “Today’s FDA approval provides children with a single-dose antiviral option, Xofluza, to treat influenza.”
The FDA approval is based on results from two Phase III studies, miniSTONE-2 and BLOCKSTONE. miniSTONE-2 evaluated Xofluza compared with oseltamivir in otherwise healthy children and included patients aged five to less than 12 years with an influenza infection and displaying influenza symptoms for no more than 48 hours. BLOCKSTONE evaluated Xofluza compared with placebo as a preventive treatment for household members (adults and children) who were living with someone with influenza.
About Genentech
Founded more than 40 years ago, Genentech is a leading biotechnology company that discovers, develops, manufactures and commercializes medicines to treat patients with serious and life-threatening medical conditions. The company, a member of the Roche Group, has headquarters in South San Francisco, California.
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules.
-
- Weblink: https://chemroboticspharma.com/pharmVetPat


