Summary :
- Five Prime’s lead asset, Bemarituzumab, is a first-in-class, Phase 3 ready anti-FGFR2b antibody with positive data from a randomized, placebo-controlled Phase 2 study in frontline advanced gastric or gastroesophageal junction (GEJ) cancer. Bemarituzumab targets FGFR2b, which has been found to be overexpressed in approximately 30% of patients with non-HER2 positive gastric cancer, as well as other solid tumors.
- Acquisition Includes Bemarituzumab, A Phase 3 Ready, First-In-Class Program For Gastric Cancer, the Third Leading Cause of Cancer Mortality Worldwide
- Bemarituzumab is a Strong Strategic Fit With Amgen’s Innovative Oncology Portfolio
- This correlation suggests that FGFR2b could play a role in other epithelial cancers, including lung, breast, ovarian and other cancers.
- The acquisition of Five Prime also supports Amgen’s international expansion strategy. Gastric cancer is one of the world’s most common forms of cancer and is particularly prevalent in the Asia-Pacific region, where Amgen expects to generate significant volume growth in the coming years. Amgen plans to leverage its presence in Japan and other Asia-Pacific markets to maximize bemarituzumab’s potential. In addition, as part of this transaction, Amgen will receive a royalty percentage on future net sales in Greater China ranging from the high teens to the low twenties from a pre-existing co-development and commercialization agreement between Five Prime and Zai Lab (Shanghai) Co., Ltd.
- Five Prime’s additional innovative pipeline programs complement Amgen’s efforts to bring meaningful therapies to oncology patients.
That drug, bemarituzumab, has been through a phase 2 trial as a front-line treatment in patients with advanced gastric and gastroesophageal junction cancers, with make-or-break data out last November.
The readout broke down like this: Investigators enrolled 155 people with FGFR2b-positive tumors, a subpopulation that accounts for 30% of all non-HER2-positive patients, and randomized them to receive bemarituzumab or placebo on top of chemotherapy.
About Bemarituzumab
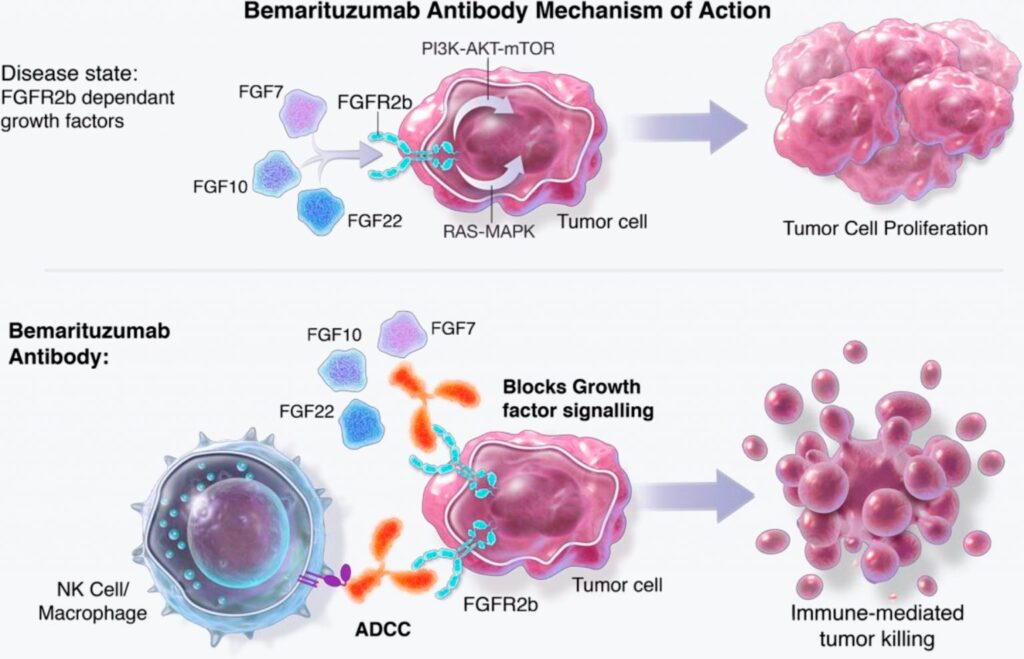
Bemarituzumab (anti-FGFR2b) is a potential first-in-class investigational targeted antibody that is designed to block fibroblast growth factors (FGFs) from binding and activating FGFR2b, inhibiting several downstream pro-tumor signaling pathways and potentially slowing cancer progression. Bemarituzumab is being developed in gastric and GEJ cancer as a targeted therapy for tumors that overexpress FGFR2b. The company is also evaluating the potential for bemarituzumab in other cancers that overexpress FGFR2b.
Indication : Gastric Cancer Treatment
Mechanism of Action
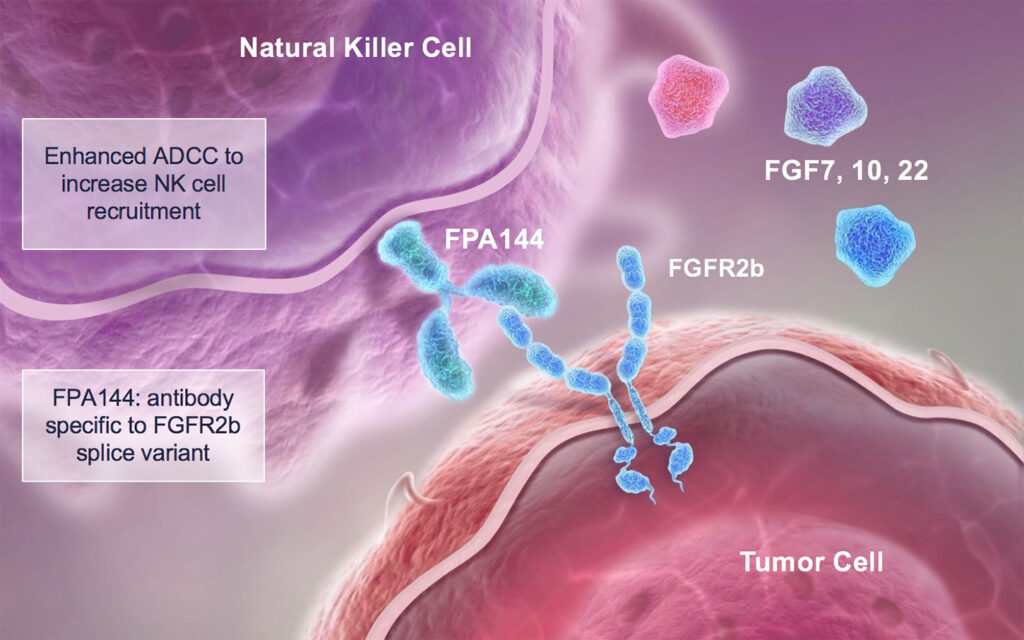
Bemarituzumab (FPA144) is a humanized monoclonal IgG1 antibody against fibroblast growth factor receptor 2b (FGFR2b, FGFR2IIIb). Bemarituzumab binds and inhibits FGFR2b on the surface of malignant cells thereby preventing FGFR2b from binding to its ligands, fibroblast growth factors 7, 10, and 22 (FGF7, FGF10, and FGF22), which promote tumor growth.
Bemarituzumab MOA
FGFR2b is a specific isoform of the receptor tyrosine kinase FGFR2 that is overexpressed in various types of cancer and is responsible for tumor proliferation, differentiation, and survival.
FGFR2b expression is observed, in addition to adenocarcinoma of the stomach or gastroesophageal junction (approximately 29% of cases), in non-small cell lung cancer (31%), triple-negative breast cancer (13%), ovarian cancer (40%), endometrial cancer (86%), cervical cancer (80%), colorectal cancer (62%), intrahepatic cholangiocarcinoma (22%), pancreatic cancer (4%).
Bemarituzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) against FGFR2b-expressing tumor cells. The result is inhibition of cell proliferation and apoptosis of malignant cells. Due to the modified glycosylation scheme (afucosylation) bemarituzumab exhibits increased affinity to Fc gamma receptor IIIA (FcγRIIIA, CD16a): since the latter is expressed by natural killer cells (NK cells) and macrophages, ADCC and local cytokine release processes are enhanced.
MOA of Bemarituzumab
The trial cleared the pre-specified bar for statistical significance against all three efficacy endpoints, although the p-values were higher than the 0.05 that typically serves as the cutoff for effectiveness.
Progression-free survival in patients who received bemarituzumab on top of chemotherapy was 9.5 months, more than two months longer than the figure in the control arm. The difference worked out at a p-value of 0.073 and a hazard ratio of 0.68. Ahead of the data readout, Five Prime Chief Medical Officer Helen Collins, M.D., told investors previously successful gastric cancer trials achieved hazard ratios of around 0.7.
As of the cutoff, Five Prime was yet to reach the median overall survival in the bemarituzumab arm. The median overall survival in the control group was 12.9 months, leading Five Prime to calculate the hazard ratio at 0.58. Roche’s Herceptin and Bristol Myer Squibb’s Opdivo, respectively, drove 2.7 and 3.3 month improvements in overall survival, resulting in hazard ratios of 0.74 and 0.71. Five Prime also saw improvements in response rate that are in line with the results achieved by both pharmas’ drugs.
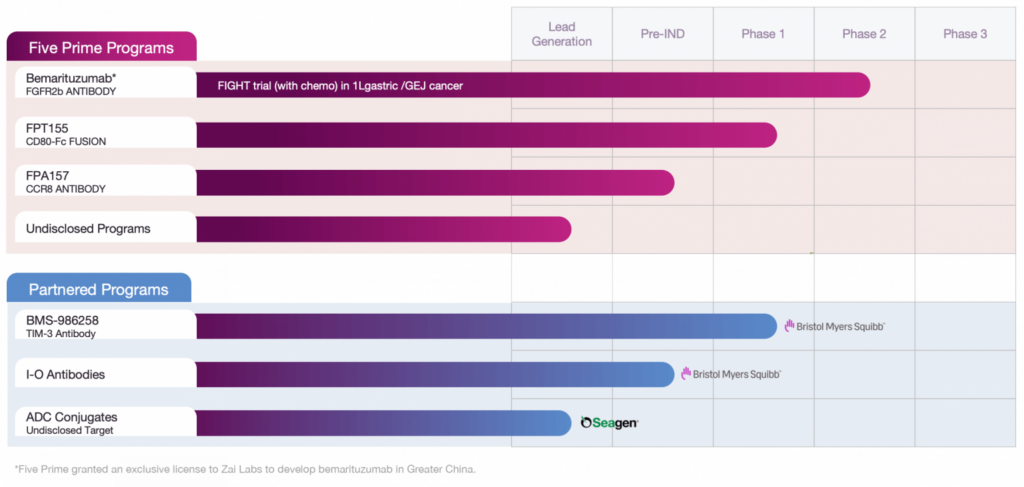
Five Prime Therapeutics: Drug Pipeline
Besides bemarituzumab, Five Prime has been developing other immuno-oncology experimental drugs. Yes, the incredible clinical success generated by the advent of PD-(L)1 and CTLA-4 blockers has made effective cancer treatment possible. Alas, many patients do not respond to these immune checkpoint inhibitors, and therefore there is a high unmet medical need for new drugs targeting other immunosuppressive mechanisms by which tumors evade immunological responses.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its expertise to strive for solutions that improve health outcomes and dramatically improve people’s lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world’s leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.


