Summary:-
- Ferring’s novel adenovirus vector-based gene therapy Adstiladrin® (nadofaragene firadenovec-vncg) is the first gene therapy approved for bladder cancer
- Efficacy and safety of Adstiladrin supported by Phase 3 results demonstrating that more than half of patients (51% of CIS ± Ta/T1 cohort) achieved a complete response (CR) at three months and of these, 46% continued to remain free of high-grade recurrence at 12 months
- Bladder cancer is the sixth most common cancer in the U.S.; Adstiladrin provides NMIBC patients a valuable alternative compared to an invasive bladder removal surgery
Ferring Pharmaceuticals today announced the U.S. Food and Drug Administration (FDA) approved Adstiladrin® (nadofaragene firadenovec-vncg), a novel adenovirus vector-based gene therapy, for the treatment of adult patients with high-risk, Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors.

FDA Approves Adstiladrin , First Gene Therapy for the Treatment of High-risk, Non-muscle-invasive bladder cancer to Ferring Pharmaceuticals
About Adstiladrin® (nadofaragene firadenovec-vncg)
API– Nadofaragene firadenovec-vncg
Description:-Adstiladrin® (nadofaragene firadenovec-vncg) is a gene therapy developed as a treatment for adult patients with BCG-unresponsive NMIBC. It is a non-replicating adenovirus vector-based gene therapy containing the gene interferon alfa-2b, administered by catheter into the bladder once every three months. The vector enters the cells of the bladder wall, releasing the active gene to do its work. The internal gene/DNA machinery of the cells “picks up” the gene and translates its DNA sequence, resulting in the cells secreting high quantities of interferon alfa-2b protein, a naturally occurring protein the body uses to fight cancer. This novel gene therapy approach thereby turns the patient’s own bladder wall cells into interferon microfactories, enhancing the body’s natural defenses against the cancer. Nadofaragene firadenovec-vncg has been studied in a clinical trial program that includes 221 patients with high-grade, BCG-unresponsive NMIBC who had been treated with adequate BCG previously and did not see benefit from additional BCG treatment (full inclusion criteria published on clinicaltrials.gov: NCT02773849)
Class –Antineoplastics; IFNA2B gene therapies
Mechanism of Action-Gene transference; IFNA2B expression stimulants
Adstiladrin MOA
CONTRAINDICATIONS
ADSTILADRIN is contraindicated in patients with hypersensitivity to interferon alfa or any component of the product.
WARNINGS AND PRECAUTIONS
- Delaying cystectomy could lead to the development of metastatic bladder cancer, which can be lethal.
- Risk of disseminated adenovirus infection: Persons who are immunocompromised or immunodeficient may be at risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus. Avoid ADSTILADRIN exposure to immunocompromised or immunodeficient individuals.
ADVERSE REACTIONS
The most common (>10%) adverse reactions, including laboratory abnormalities (>15%), were glucose increased (38%), instillation site discharge (33%), triglycerides increased (30%), fatigue (24%), bladder spasm (20%), micturition (urination urgency) (19%), creatinine increased (17%), hematuria (blood in urine) (17%), phosphate decreased (16%), chills (16%), pyrexia (fever) (15%), and dysuria (painful urination) (16%).
New Approval
The safety and effectiveness of nadofaragene firadenovec-vncg was evaluated in a multicenter clinical study that included 157 patients with high-risk BCG-unresponsive non–muscle-invasive bladder cancer, 98 of whom had BCG-unresponsive carcinoma in situ with or without papillary tumors and could be evaluated for response. Patients received nadofaragene firadenovec-vncg once every 3 months for up to 12 months, or until unacceptable toxicity or recurrent disease.
Overall, 51% of enrolled patients receiving nadofaragene firadenovec-vncg achieved a complete response. The median duration of response was 9.7 months. About 46% of responding patients remained in complete response for at least 1 year.
The most common adverse reactions associated with nadofaragene firadenovec-vncg included bladder discharge, fatigue, bladder spasm, urinary urgency, hematuria, chills, fever, and painful urination. Individuals who are immunosuppressed or immune-deficient should not come into contact with nadofaragene firadenovec-vncg.
Nadofaragene firadenovec-vncg is administered once every 3 months into the bladder via a urinary catheter.
This application was granted Priority Review, Breakthrough Therapy, and Fast Track designations.
About Non-Muscle Invasive Bladder Cancer (NMIBC)
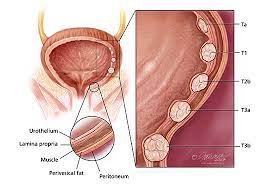
Non-Muscle Invasive Bladder Cancer (NMIBC)
NMIBC is a form of bladder cancer which is present in the superficial layer of the bladder and has not invaded deeper into the bladder or spread to other parts of the body. Bladder cancer is the sixth most common cancer in the U.S., and it is estimated that there were approximately 81,180 new cases of bladder cancer in the U.S. in 2022, 75% of which present as NMIBC. In patients with high-risk NMIBC, intravesical BCG remains the first-line standard of care. However, more than 50% of patients who receive initial treatment with BCG will experience disease recurrence and progression within one year, with many developing BCG-unresponsive disease.Current treatment options for BCG-unresponsive patients are very limited, and often result in a highly invasive life-changing procedure of radical cystectomy (complete removal of the bladder).
About the Phase 3 Study
The Phase 3 study of nadofaragene firadenovec-vncg in 157 patients from 33 U.S. sites met its primary endpoint with more than half (51%) of the 98 patients (95% CI 41 to 61) with carcinoma in situ with or without concomitant high-grade Ta or T1 disease (CIS ± Ta/T1) achieving a complete response (CR), all by three months. Of the patients who achieved an initial CR, 46% (n=23 of 50) continued to remain free of high-grade recurrence at 12 months. In the study, nadofaragene firadenovec-vncg was administered directly into the patient’s bladder by instillation once every three months by a healthcare professional.
The most common adverse events (AEs) observed in the study that occurred in patients in order of decreasing frequency were: instillation site discharge (33%), fatigue (24%), bladder spasm (20%), micturition urgency (19%), and hematuria (17%). The discontinuation rate due to AEs was 1.9%.
The long-term follow-up phase of the four-year study is ongoing, and patients are continuing to be monitored for a total of five years.
Bladder cancer, one of the more common forms of cancer, is a disease in which malignant (cancer) cells form a tumor in the tissues of the bladder. These abnormal cells can invade and destroy normal body tissue. Over time, the abnormal cells can also metastasize (spread) through the body. Most newly diagnosed bladder cancers (75% to 80%) are classified as NMIBC – a type of cancer that has grown through the lining of the bladder but hasn’t yet invaded the muscle layer. This type of cancer is associated with high rates of recurrence (between 30 to 80%) and the risk of progression to invasive and metastatic cancer.
Treatment and care of patients with high-risk NMIBC, including those with carcinoma in situ, or CIS (abnormal cancer cells found in the place where they first formed and that have not spread to nearby tissue), often involves removing the tumor and the use of BCG to reduce the risk that the cancer will recur. Few effective treatment options exist for patients who develop BCG-unresponsive disease. The failure to achieve a complete response, or the disappearance of all signs of cancer as seen on cystoscopy, biopsied tissue, and urine, is associated with an increased risk of death or a disease-worsening event. Without treatment, the cancer can invade, damage tissues and organs, and spread through the body. According to the Centers for Disease Control and Prevention, about 57,000 men and 18,000 women are diagnosed with bladder cancer annually, and roughly 12,000 men and 4,700 women die from the disease each year in the United States.
About Ferring Pharmaceuticals
Ferring Pharmaceuticals is a Swiss multinational biopharmaceutical company specialising in areas such as reproductive health, maternal health, gastroenterology and urology. Ferring has been developing treatments for mothers and babies for over 50 years. Founded in 1950, privately-owned Ferring employs more than 5,400 people worldwide (6,500 in 2017), has its own operating subsidiaries in nearly 60 countries and markets its products in 110 countries.
PharmVetPat® - Pharma || Veterinary || Agrochemicals Active Ingredients and their mixtures with more than 30 physicochemical properties. Users can access the full product landscape report comprising chemistry, including route of synthesis, biology, IP (Patents including Compound Patent / Innovator / Developer Info.) and regulatory info. with product identification including CAS No., developmental code, EPA Codes. Compound and intermediate synthetic routes with raw material including Key Starting Material (KSM) or Intermediate Information. Territory Coverage: USA, Europe, Canada, India, Japan and Korea. View Pharma Database

