Summary:
Food and Drug Administration approved fam-trastuzumab deruxtecan-nxki (Enhertu, Daiichi Sankyo, Inc.) for adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within 6 months of completing therapy.
About Fam-Trastuzumab Deruxtecan-Nxki
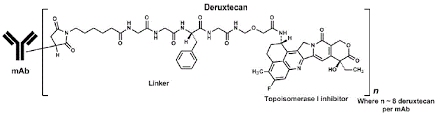
Fam-Trastuzumab Deruxtecan-Nxki Structure
- US Brand Name :Enhertu
- Fam-trastuzumab deruxtecan-nxki injection is used to treat HER2-positive metastatic (cancer that has spread to other parts of the body) breast cancer or whose cancer cannot be removed with surgery in patients who have previously received two or more anti-HER2-based regimens in the metastatic setting.
- Medical Use : ENHERTU is a prescription medicine used in adults to treat human epidermal growth factor receptor 2 (HER2)-positive: Breast cancer that cannot be removed by surgery or that has spread to other parts of your body (metastatic), and who have received two or more prior anti-HER2 breast cancer treatments.
Enhertu Mechanism of Action
ENHERTU binds to HER2-receptors expressed on tumor cells to form an ENHERTU-HER2 complex, which is internalized. After internalization, the linker is selectively cleaved by lysosomal enzymes that are upregulated in tumor cells, releasing the payload inside the cancer cells.
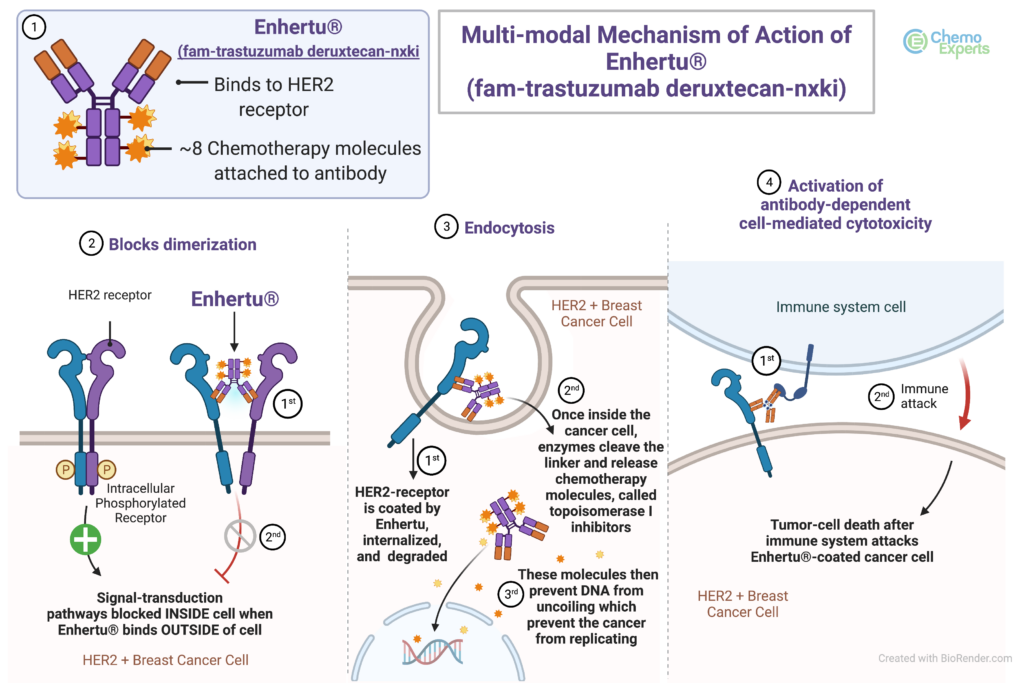
Enhertu mechanism of action
DESTINY-Breast03
Efficacy was based on DESTINY-Breast03, a multicenter, open-label, randomized trial that enrolled 524 patients with HER2-positive, unresectable, and/or metastatic breast cancer who received prior trastuzumab and taxane therapy for metastatic disease or developed disease recurrence during or within 6 months of completing neoadjuvant or adjuvant therapy. Patients were randomly assigned 1:1 to receive either fam-trastuzumab deruxtecan-nxki or ado-trastuzumab emtansine by intravenous infusion every 3 weeks until unacceptable toxicity or disease progression. Random assignment was stratified by hormone receptor status, prior treatment with pertuzumab, and history of visceral disease.
The main efficacy outcome measure was progression-free survival as assessed by blinded independent central review based on Response Evaluation Criteria in Solid Tumors version 1.1. Overall survival and confirmed objective response rate were the key secondary outcome measures.
Median progression-free survival was not reached (95% confidence interval [CI] = 18.5 months–not estimable) in the fam-trastuzumab deruxtecan-nxki arm and 6.8 months (95% CI = 5.6–8.2 months) in the ado-trastuzumab emtansine arm. The hazard ratio was 0.28 (95% CI = 0.22–0.37, P < .0001). At the time of the progression-free survival analysis, 16% of patients had died and overall survival data was immature. The objective response rate based on the patients with measurable disease assessed by blinded independent central review at baseline was 82.7% (95% CI = 77.4%–87.2%) in the fam-trastuzumab deruxtecan-nxki arm and 36.1% (95% CI = 30.0%–42.5%) for those receiving ado-trastuzumab emtansine.
The most common adverse reactions (incidence > 30%) in patients receiving fam-trastuzumab deruxtecan-nxki were nausea, fatigue, vomiting, alopecia, constipation, anemia, and musculoskeletal pain. Serious adverse reactions in > 1% of patients who received fam-trastuzumab deruxtecan-nxki were vomiting, interstitial lung disease, pneumonia, pyrexia, and urinary tract infection. The prescribing information includes a boxed warning to advise health professionals of the risk of interstitial lung disease and embryofetal toxicity.
The recommended fam-trastuzumab deruxtecan-nxki dose for breast cancer is 5.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity.


