Summary :
- Pfizer Inc. and Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) today announced that the European Commission (EC) has granted marketing authorization for VYDURA® (rimegepant), a calcitonin gene-related peptide (CGRP) receptor antagonist for both the acute treatment of migraine with or without aura, and prophylaxis of episodic migraine in adults who have at least four migraine attacks per month.
- VYDURA®, an orally disintegrating tablet, is the first medicine approved for both acute and prophylactic treatment of migraine in the European Union (EU).
- Migraine is a leading cause of disability worldwide with approximately one in ten people living with the condition in Europe alone. Globally, migraine disproportionately affects women by three to four times compared to men.
About VYDURA® (Rimegepant)
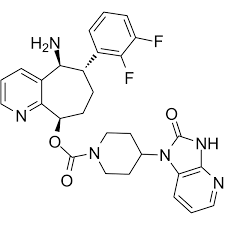
Rimegepant Structure
VYDURA® targets a key component of migraine by reversibly blocking CGRP receptors. CGRP is increased during a migraine attack, dilates blood vessels and is involved in nociceptor signaling. CGRP receptor antagonists work by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the endogenous CGRP neuropeptide.
- API-Rimegepant
- Indication– Rimegepant is currently the only migraine medication approved to both treat acute migraine attacks and help prevent future migraine attacks. The new indication allows for use of rimegepant for preventive treatment in adults with episodic migraine (> 15 migraine days per month)
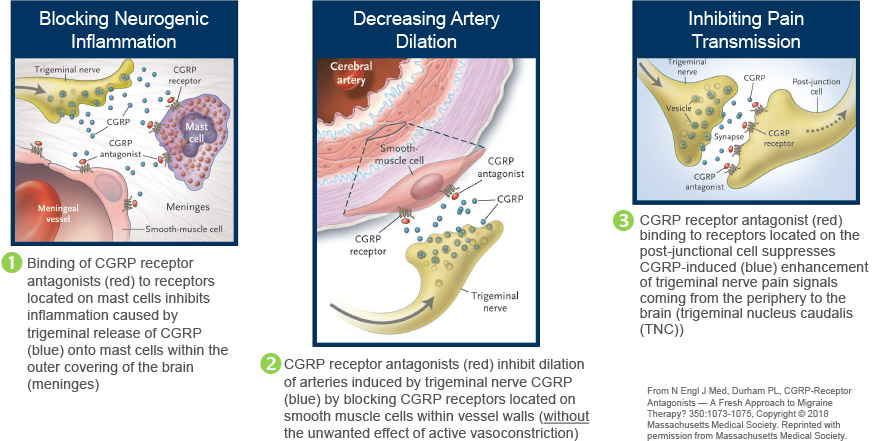
Rimegepant Mechanism of Action
“There is a significant unmet need for people in the European Union living with the pain and disability caused by frequent migraines,” said Nick Lagunowich, Global President, Pfizer Internal Medicine. “The comprehensive clinical program has established VYDURA’s efficacy and safety as both an acute and preventive treatment of migraine. Studies in acute migraine demonstrated a rapid and long-lasting relief of migraine headache and other symptoms with a single dose, while the prevention study found a significant reduction in migraine attacks with every other day dosing. We have great confidence in the positive impact VYDURA could have on people living with this debilitating condition in the EU.”
Results from the Phase 3 study published in Lancet demonstrated that a single dose of rimegepant provided superior pain reduction and associated symptoms of migraine at two hours compared to placebo. The prevention study, also published in Lancet, demonstrated that rimegepant taken every other day provided superior reduction in the number of days per month with migraine in Weeks 9 – 12 of the 12-week treatment period compared to placebo, that was maintained with continued dosing during the 12-month open-label extension period.
“Today’s approval marks a huge step forward for patients in Europe who are living with migraine. Migraine is often overlooked and undertreated, resulting in substantial disability with suboptimal care for patients,” commented Professor Peter Goadsby, Director of the National Institute for Health and Care Research (NIHR) Clinical Research Facility and Professor of Neurology at King’s College London. “VYDURA’s promising efficacy and favorable benefit-risk profile spark hope for people in need of new migraine treatment options. This approval has the potential to advance the standard of care for migraine in the EU and I am hopeful it will improve the quality of life for many people living with the burden of this prevalent neurological disease.”
The Marketing Authorization follows the recommendation for approval by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in February. The EC approval will be valid for all 27 EU member states as well as Iceland, Liechtenstein, and Norway and local reimbursement approval will follow. Assessment of the marketing authorization application by the Medicines & Healthcare products Regulatory Agency (MHRA) is underway and approval is expected to shortly follow in the United Kingdom.
About Migraine
More than one billion people worldwide suffer from migraine. Migraine is characterized by debilitating attacks lasting four to 72 hours with multiple symptoms, including pulsating headaches of moderate to severe pain intensity that can be associated with nausea or vomiting, and/or sensitivity to sound (phonophobia) and sensitivity to light (photophobia). There is a significant unmet need for new treatments as more than 90 per cent of people with migraine are unable to work or function normally during an attack.


