Summary :
Camzyos (mavacamten), the first FDA-approved cardiac myosin inhibitor that directly addresses the root of obstructive hypertrophic cardiomyopathy, has been authorised by the US Food and Drug Administration (HCM).
Obstructive HCM is a progressive disease where the heart walls thicken, which increases the difficulty for the heart to expand normally and fill with blood. Mechanistic hallmarks of Obstructive HCM are excess myosin actin cross-bridge formation and dysregulation of the super-relaxed state.
About Camzyos (Mavacamten)
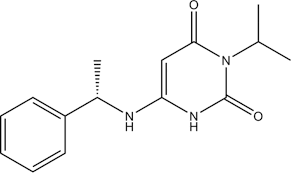
Mavacamten Structure
API -Mavacamten
Indication – Mavacamten is indicated for the treatment of adults with symptomatic New York Heart Association class II-III obstructive hypertrophic cardiomyopathy to improve functional capacity and symptoms
Mechanism of Action:
Mavacamten, formerly known as MYK-461 is a recently discovered novel small-molecule modulator of cardiac myosin that targets the underlying sarcomere hypercontractility of hypertrophic cardiomyopathy, one of the most prevalent heritable cardiovascular disorders. Studies on isolated cells and muscle fibers as well as intact animals have shown that mavacamten inhibits sarcomere force production, thereby reducing cardiac contractility. Initial mechanistic studies have suggested that mavacamten primarily reduces the steady-state ATPase activity by inhibiting the rate of phosphate release of β-cardiac myosin-S1, but the molecular mechanism of action of mavacamten has not been described.

MOA
About Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a chronic, progressive disease in which excessive contraction of the heart muscle and reduced ability of the left ventricle to fill can lead to the development of debilitating symptoms and cardiac dysfunction. HCM is estimated to affect one in every 500 people globally.
The most frequent cause of HCM is mutations in the heart muscle proteins of the sarcomere. In both obstructive and non-obstructive HCM patients, exertion can result in fatigue or shortness of breath, interfering with a patient’s ability to participate in activities of daily living. HCM has also been associated with increased risks of atrial fibrillation, stroke, heart failure and sudden cardiac death.
About LianBio
LianBio is a cross-border biotechnology company on a mission to bring transformative medicines to historically underserved patients in China and other Asian markets. Through partnerships with highly innovative biopharmaceutical companies around the world, LianBio is advancing a diversified portfolio of clinically validated product candidates with the potential to drive new standards of care across cardiovascular, oncology, ophthalmology, inflammatory disease and respiratory indications. LianBio is establishing an international infrastructure to position the company as a partner of choice with a platform to provide access to China and other Asian markets.
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)


