Summary :
- Third approved indication for SKYRIZI (risankizumab-rzaa) is supported by safety and efficacy data from two induction and one maintenance clinical trials evaluating SKYRIZI in moderately to severely active Crohn’s disease, ADVANCE, MOTIVATE and FORTIFY.
- As early as week 4 in the induction studies, clinical response and clinical remission were achieved by significantly more subjects treated with SKYRIZI versus placebo, as were co-primary endpoints of endoscopic response and clinical remission at week 12 and week 52.
- Crohn’s disease is chronic, systemic and progressive; most patients experience unpredictable symptoms that have a significant impact on daily life
AbbVie (NYSE: ABBV) today announced that the U.S. Food and Drug Administration (FDA) has approved SKYRIZI® (risankizumab-rzaa) as the first and only specific interleukin-23 (IL-23) inhibitor for the treatment of adults with moderately to severely active Crohn’s disease (CD). In two induction and one maintenance clinical trials, SKYRIZI demonstrated significant improvements in endoscopic response (defined as a decrease of greater than 50% from the baseline Simple Endoscopic Score in CD [SES-CD] or for patients with isolated ileal disease and SES-CD of 4, at least a 2-point reduction from baseline) and clinical remission (defined as a Crohn’s Disease Activity Index [CDAI] of less than 150), compared to placebo, as both an induction and maintenance therapy
“We are proud to offer the first new treatment option in six years for moderately to severely active CD, which may provide patients with a meaningful level of endoscopic improvement,” said Thomas Hudson, M.D., senior vice president, research and development, chief scientific officer, AbbVie. “With more than 30 ongoing or planned trials in inflammatory bowel disease, AbbVie is committed to advancing the standards of care for patients by exploring and investing in research for those living with immune-mediated, gastroenterological conditions.”
About SKYRIZI® (risankizumab-rzaa)
Risankizumab, Monoclonal Antibodiy Structure
- API – Risankizumab-rzaa
- Description : Risankizumab-rzaa injection is used to treat moderate to severe plaque psoriasis in patients who may benefit from receiving phototherapy (ultraviolet light treatment) or other treatments. Plaque psoriasis is a skin disease with red patches and white scales that do not go away.
- Indication-SKYRIZI is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
- Dosing – The dosing regimen for SKYRIZI for the treatment of CD is 600 mg administered by intravenous infusion over at least one hour at week 0, week 4, and week 8, followed by 360 mg self-administered by subcutaneous injection (SC) with an on-body injector (OBI) at week 12, and every 8 weeks thereafter. A 180 mg self-administered SC maintenance dose option remains under review by the FDA.
SKYRIZI may cause serious side effects, including:
- Serious allergic reactions: Stop using SKYRIZI and get emergency medical help right away if you get any symptoms of a serious allergic reaction.
- Infections: SKYRIZI may increase your risk of infections. Before starting treatment, your doctor should check you for infections and tuberculosis. Tell your doctor right away if you have an infection or symptoms of one.
About Crohn’s Disease
Crohn’s disease is a chronic, systemic disease that manifests as inflammation within the gastrointestinal (or digestive) tract, causing persistent diarrhea and abdominal pain. It is a progressive disease, meaning it gets worse over time, and in many cases leads to surgery. Because the signs and symptoms of Crohn’s disease are unpredictable, it causes a significant burden on people living with the disease.
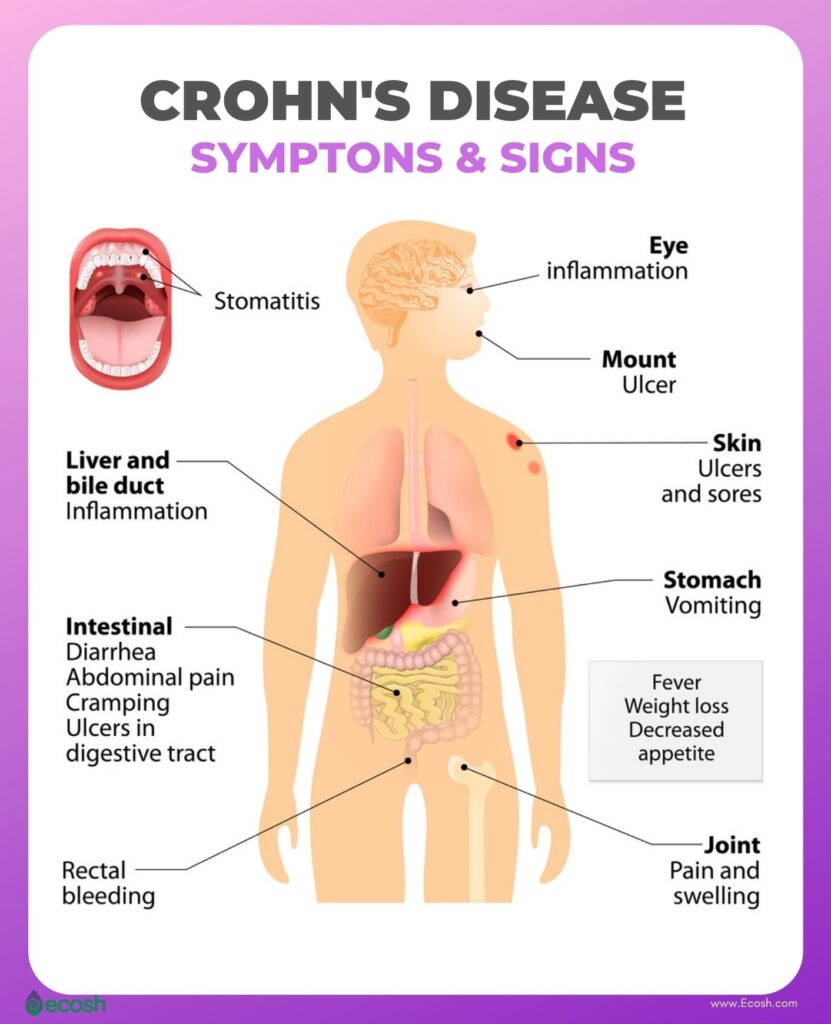
Crohn’s disease Symptoms
Crohn’s disease can cause abdominal pain, diarrhoea, weight loss, anaemia and fatigue. Some people may be symptom-free most of their lives, while others can have severe chronic symptoms that never go away.
About the FORTIFY Study
The FORTIFY study is a Phase 3, multicenter, randomized, double-blind, control group, 52-week maintenance study designed to evaluate the efficacy and safety of risankizumab 180 mg and 360 mg as maintenance therapy versus withdrawal in patients who responded to risankizumab induction treatment in the ADVANCE and MOTIVATE studies. This study included different sets of primary and secondary endpoints for the OUS analysis plan and U.S. analysis plan due to regulatory requirements in the different regions. The co-primary endpoints were achievement of endoscopic response and clinical remission at week 52. Endoscopic response is defined as a decrease of greater than 50% from the baseline SES-CD or for patients with isolated ileal disease and SES-CD of 4, at least a 2-point reduction from baseline, as scored by a central reviewer. Clinical remission is defined by SF/AP, which was measured by daily stool frequency and abdominal pain score, in the OUS analysis plan and defined by CDAI, which was measured by a CDAI score less than 150, in the U.S. analysis plan.
About AbbVie in Gastroenterology
With a robust clinical trial program, AbbVie is committed to cutting-edge research to drive exciting developments in inflammatory bowel diseases (IBD), like ulcerative colitis and Crohn’s disease. By innovating, learning and adapting, AbbVie aspires to eliminate the burden of IBD and make a positive long-term impact on the lives of people with IBD.
About AbbVie
AbbVie’s mission is to discover and deliver innovative medicines that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people’s lives across several key therapeutic areas: immunology, oncology, neuroscience, eye care, virology, women’s health and gastroenterology, in addition to products and services across our Allergan Aesthetics portfolio.
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules.
-


