Summary :
- Amgen today announced that the U.S. Food and Drug Administration (FDA) has approved RIABNI™ (rituximab-arrx), a biosimilar to Rituxan®, in combination with methotrexate for adults with moderate to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more tumor necrosis factor (TNF) antagonist therapies.
- RIABNI is already approved for the treatment of adult patients with Non-Hodgkin’s Lymphoma (NHL), Chronic Lymphocytic Leukemia (CLL), Granulomatosis with Polyangiitis (GPA) (also called Wegener’s Granulomatosis) and Microscopic Polyangiitis (MPA).
“The approval of RIABNI is an important advancement for adults living with moderate to severe rheumatoid arthritis, a chronic inflammatory joint disease, who now have access to a proven and affordable treatment option,” said Murdo Gordon, executive vice president of Global Commercial Operations at Amgen. “Our fully integrated portfolio of innovative and biosimilar medicines for inflammatory diseases reinforces our commitment to providing patients with high-quality and affordable treatment options that deliver substantial value to our healthcare system.”
About RIABNI
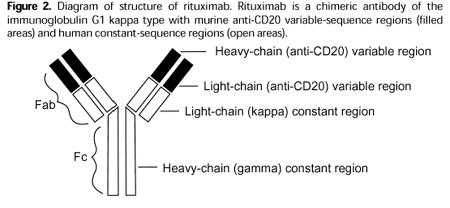
RIABNI STRUCTURE
- Adults with Non-Hodgkin’s Lymphoma (NHL): Alone or with other chemotherapy medicines
- Adults with Chronic Lymphocytic Leukemia (CLL): With the chemotherapy medicines fludarabine and cyclophosphamide
API– Rituximab-arrx
RIABNI is a biosimilar to Rituxan, an anti-CD20 monoclonal antibody. The active ingredient of RIABNI is a monoclonal antibody that has the same amino acid sequence as Rituxan. RIABNI also has the same strength as Rituxan, and the dosage form and route of administration are identical to Rituxan. RIABNI is not currently indicated as a treatment for children with mature B-cell Non-Hodgkin’s lymphoma, mature B-cell acute leukemia, MPA, or GPA. RIABNI is not indicated in adult patients with moderate to severe pemphigus vulgaris (PV), for which Rituxan has orphan status.
Mechanism of Action
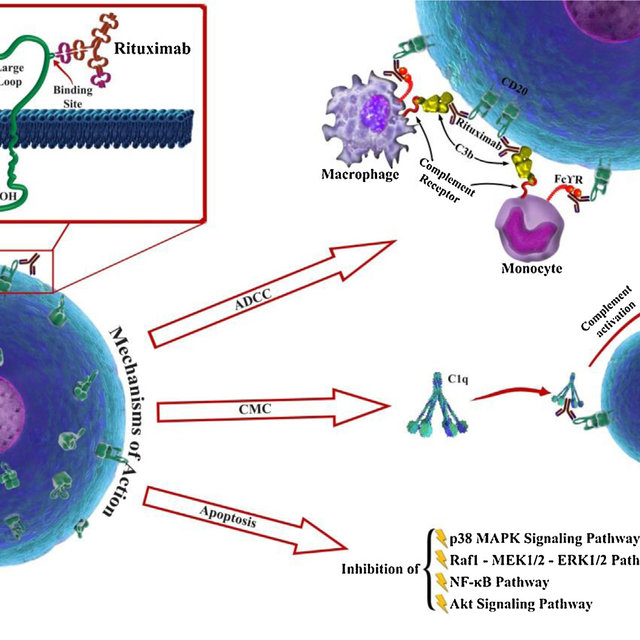
RIABNI MOA
IMPORTANT SAFETY INFORMATION AND INDICATIONS
RIABNI™ can cause serious side effects that can lead to death, including:
-
- Infusion-Related Reactions: Infusion-related reactions are very common side effects of RIABNI™ treatment. Serious infusion-related reactions can happen during your infusion or within 24 hours after your infusion of RIABNI™. Your healthcare provider should give you medicines before your infusion of RIABNI™ to decrease your chance of having a severe infusion-related reaction.
Tell your healthcare provider or get medical help right away if you get any of these symptoms during or after an infusion of RIABNI™:
- Hives (red itchy welts) or rash
- Itching
- Swelling of your lips, tongue, throat, or face
- Sudden cough
- Shortness of breath, difficulty breathing, or wheezing
- Weakness
- Dizziness or feel faint
- Palpitations (feel like your heart is racing or fluttering)
- Chest pain
- Severe Skin and Mouth Reactions: Tell your healthcare provider or get medical help right away if you get any of these symptoms at any time during your treatment with RIABNI™:
- Painful sores or ulcers on your skin, lips, or in your mouth
- Blisters
- Peeling skin
- Rash
- Pustules
- Hepatitis B Virus (HBV) Reactivation: Before you receive your RIABNI™ treatment, your healthcare provider will do blood tests to check for HBV infection. If you have had hepatitis B or are a carrier of the hepatitis B virus, receiving RIABNI™ could cause the virus to become an active infection again. Hepatitis B reactivation may cause serious liver problems, including liver failure, and death. You should not receive RIABNI™ if you have active hepatitis B liver disease. Your healthcare provider will monitor you for hepatitis B infection during and for several months after you stop receiving RIABNI™.
Tell your healthcare provider right away if you get worsening tiredness, or yellowing of your skin or white part of your eyes during treatment with RIABNI™.
- Progressive Multifocal Leukoencephalopathy (PML): PML is a rare, serious brain infection caused by a virus that can happen in people who receive RIABNI™. People with weakened immune systems can get PML. PML can result in death or severe disability. There is no known treatment, prevention, or cure for PML.
Tell your healthcare provider right away if you have new or worsening symptoms or if anyone close to you notices these symptoms:
- Confusion
- Dizziness or loss of balance
- Difficulty walking or talking
- Decreased strength or weakness on one side of your body
- Vision problems, such as blurred vision or loss of vision
- Infusion-Related Reactions: Infusion-related reactions are very common side effects of RIABNI™ treatment. Serious infusion-related reactions can happen during your infusion or within 24 hours after your infusion of RIABNI™. Your healthcare provider should give you medicines before your infusion of RIABNI™ to decrease your chance of having a severe infusion-related reaction.
Before receiving RIABNI™, tell your healthcare provider if you:
-
- Have had a severe reaction to RIABNI™ or a rituximab product
- Have a history of heart problems, irregular heartbeat, or chest pain
- Have lung or kidney problems
- Have had an infection, currently have an infection, or have a weakened immune system
- Have or have had any severe infections including:
- Hepatitis B virus (HBV)
- Hepatitis C virus (HCV)
- Cytomegalovirus (CMV)
- Herpes simplex virus (HSV)
- Parvovirus B19
- Varicella zoster virus (chickenpox or shingles)
- West Nile Virus
- Have had a recent vaccination or are scheduled to receive vaccinations. You should not receive certain vaccines before or during treatment with RIABNI™.
- Have any other medical conditions
- Are pregnant or plan to become pregnant. Talk to your healthcare provider about the risks to your unborn baby if you receive RIABNI™ during pregnancy. Females who are able to become pregnant should use effective birth control (contraception) during treatment with RIABNI™ and for at least 12 months after the last dose of RIABNI™. Talk to your healthcare provider about effective birth control. Tell your healthcare provider right away if you become pregnant or think that you are pregnant during treatment with RIABNI™.
- Are breastfeeding or plan to breastfeed. It is not known if RIABNI™ passes into your breast milk. Do not breastfeed during treatment and for at least 6 months after your last dose of RIABNI™.
- Are taking any medications, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take or have taken:
- A tumor necrosis factor (TNF) inhibitor medicine
- A disease modifying anti-rheumatic drug (DMARD)
If you are not sure if your medicine is one listed above, ask your healthcare provider.
RIABNI™ can cause serious side effects, including:
- Tumor Lysis Syndrome (TLS): TLS is caused by the fast breakdown of cancer cells. TLS can cause you to have:
- Kidney failure and the need for dialysis treatment
- Abnormal heart rhythm
TLS can happen within 12 to 24 hours after an infusion of RIABNI™. Your healthcare provider may do blood tests to check you for TLS. Your healthcare provider may give you medicine to help prevent TLS. Tell your healthcare provider right away if you have any of the following signs or symptoms of TLS:
- Nausea
- Vomiting
- Diarrhea
- Lack of energy
- Serious Infections: Serious infections can happen during and after treatment with RIABNI™, and can lead to death. RIABNI™ can increase your risk of getting infections and can lower the ability of your immune system to fight infections. Types of serious infections that can happen with RIABNI™ include bacterial, fungal, and viral infections. After receiving RIABNI™, some people have developed low levels of certain antibodies in their blood for a long period of time (longer than 11 months). Some of these patients with low antibody levels developed infections. People with serious infections should not receive RIABNI™. Tell your healthcare provider right away if you have any symptoms of infection:
- Fever
- Cold symptoms, such as runny nose or sore throat that do not go away
- Flu symptoms, such as cough, tiredness, and body aches
- Earache or headache
- Pain during urination
- Cold sores in the mouth or throat
- Cuts, scrapes, or incisions that are red, warm, swollen, or painful
- Heart Problems: RIABNI™ may cause chest pain, irregular heartbeats, and heart attack. Your healthcare provider may monitor your heart during and after treatment with RIABNI™ if you have symptoms of heart problems or have a history of heart problems. Tell your healthcare provider right away if you have chest pain or irregular heartbeats during treatment with RIABNI™.
- Kidney Problems: RIABNI™ can cause severe kidney problems that lead to death, especially if you are receiving RIABNI™ for Non-Hodgkin’s Lymphoma (NHL). Your healthcare provider should do blood tests to check how well your kidneys are working.
- Stomach and Serious Bowel Problems That Can Sometimes Lead to Death: Bowel problems, including blockage or tears in the bowel, can happen if you receive RIABNI™ with chemotherapy medicines. Tell your healthcare provider right away if you have any stomach-area (abdomen) pain or repeated vomiting during treatment with RIABNI™.
Common side effects of RIABNI™ include:
- Infusion-related reactions
- Infections (may include fever, chills)
- Body aches
- Tiredness
- Nausea
In adult patients with Granulomatosis with Polyangiitis (GPA) (Wegener’s Granulomatosis) or Microscopic Polyangiitis (MPA), the most common side effects of RIABNI™ also include:
- Low white and red blood cells
- Swelling
- Diarrhea
- Muscle spasms
Other side effects with RIABNI™ include:
- Aching joints during or within hours of receiving an infusion
- More frequent upper respiratory tract infections
About Amgen Biosimilars
Amgen is committed to building upon Amgen’s experience in the development and manufacturing of innovative human therapeutics to expand Amgen’s reach to patients with serious illnesses. Biosimilars help to maintain Amgen’s commitment to connect patients with vital medicines, and Amgen is well positioned to leverage its nearly four decades of experience in biotechnology to create high-quality biosimilars and reliably supply them to patients worldwide.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules.
-


