Summary:
- Approval based on superior survival outcomes of Libtayo plus chemotherapy, compared to chemotherapy alone, in a patient population with a wide range of disease characteristics
- Second advanced NSCLC indication expands patient population eligible for a Libtayo-based regimen to include combination treatment with chemotherapy irrespective of PD-L1 expression levels.
Regeneron Pharmaceuticals, Inc announced that the U.S. Food and Drug Administration (FDA) has approved the PD-1 inhibitor Libtayo® (cemiplimab-rwlc) in combination with platinum-based chemotherapy for the first-line treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with no EGFR, ALK or ROS1 aberrations. Patients must either have metastatic or locally advanced tumors that are not candidates for surgical resection or definitive chemoradiation. Patients may be treated with this combination irrespective of PD-L1 expression or histology.
The FDA approval is based on data from the global Phase 3 trial, EMPOWER-Lung 3, that investigated Libtayo in combination with a physician’s choice of platinum-doublet chemotherapy (Libtayo combination), compared to platinum-doublet chemotherapy alone. The trial enrolled 466 patients with locally advanced or metastatic NSCLC, irrespective of PD-L1 expression or tumor histology, and with no ALK, EGFR or ROS1 aberrations. Among those enrolled, 43% had tumors with squamous histology, 67% had tumors with <50% PD-L1 expression, 15% had inoperable locally advanced disease not eligible for definitive chemoradiation, and 7% had pretreated and clinically stable brain metastases.
About Libtayo
Description -Libtayo is a fully human monoclonal antibody targeting the immune checkpoint receptor PD-1 on T-cells and was invented using Regeneron’s proprietary VelocImmune® technology. By binding to PD-1, Libtayo has been shown to block cancer cells from using the PD-1 pathway to suppress T-cell activation. In the U.S. and other countries Libtayo is indicated in certain patients with advanced basal cell carcinoma (BCC), advanced cutaneous squamous cell carcinoma (CSCC) and advanced NSCLC, as well as in advanced cervical cancer in Canada and Brazil. As of July 1, 2022, Libayo is developed and marketed globally by Regeneron.
- In the U.S., the generic name for Libtayo in its approved indications is cemiplimab-rwlc, with rwlc as the suffix designated in accordance with Nonproprietary Naming of Biological Products Guidance for Industry issued by the FDA. Outside of the U.S., the generic name of Libtayo in its approved indication is cemiplimab.
- The extensive clinical program for Libtayo is focused on difficult-to-treat cancers. Libtayo is currently being investigated in trials as a monotherapy, as well as in combination with either conventional or novel therapeutic approaches for other solid tumors and blood cancers. These potential uses are investigational, and their safety and efficacy have not been evaluated by any regulatory authority.
API-Cemiplimab
Type-Biotech
Indication :-Cemiplimab is indicated to treat:
- Locally advanced or metastatic cutaneous squamous cell carcinoma (mCSCC) in patients who are not candidates for curative surgery or curative radiation.
- Locally advanced basal cell carcinoma (laBCC) in previously treated patients with a hedgehog pathway inhibitor or for whom a hedgehog pathway inhibitor is not appropriate.
- Metastatic basal cell carcinoma (mBCC) in patients who were previously treated with a hedgehog pathway inhibitor or for whom a hedgehog pathway inhibitor is not appropriate. This indication is approved under accelerated approval based on tumour response rate and durability of response. Continued approval for mBCC may be contingent upon verification and description of clinical benefit.
- Locally advanced non-small cell lung cancer (NSCLC) in combination with platinum‐based chemotherapy for the first‐line treatment of adults with no EGFR, ALK or ROS1 aberrations, who are not candidates for surgical resection or definitive chemoradiation. It is also indicated to treat metastatic NSCLC in combination with platinum‐based chemotherapy as first-line treatment in adults.
- Locally advanced or metastatic NSCLC as monotherapy for the first-line treatment of adults whose tumours have high PD-L1 expression [Tumor Proportion Score (TPS) ≥ 50%] as determined by an FDA-approved test, with no EGFR, ALK or ROS1 aberrations. Patients with locally advanced NSCLC must not be candidates for surgical resection or definitive chemoradiation.
Mechanism of Action-Antibody-dependent cell cytotoxicity; Programmed cell death-1 receptor antagonists; T lymphocyte stimulants
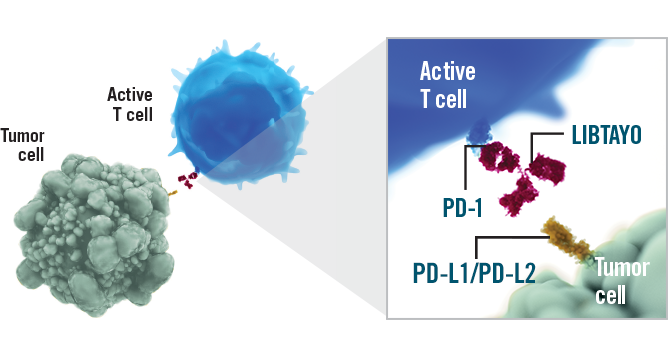
Mechanism of Action of Cemiplimab
Orphan Drug Status-Yes – Cervical cancer
Efficacy in EMPOWER-Lung 3 was assessed in 466 patients who were randomized 2:1 to receive either Libtayo 350 mg (n=312) or placebo (n=154) intravenously every 3 weeks, plus histology-specific platinum-doublet chemotherapy. The trial was stopped early based on a recommendation by the Independent Data Monitoring Committee after the Libtayo combination demonstrated a significant improvement in overall survival (OS), the primary endpoint. Efficacy results comparing the Libtayo combination to chemotherapy alone showed a:
- 22-month median OS versus 13 months, representing a 29% relative reduction in the risk of death (hazard ratio [HR]: 0.71; 95% confidence interval [CI]: 0.53 to 0.93; p=0.014). The 12-month probability of survival was 66% for the Libtayo combination versus 56% for chemotherapy, per Kaplan-Meier estimates.
- 8-month median PFS versus 5 months, representing a 44% reduction in the risk of disease progression (HR: 0.56; 95% CI: 0.44 to 0.70; p<0.0001). The 12-month probability of PFS for the Libtayo combination was 38% versus 16% for chemotherapy.
- 43% overall response rate versus 23%.
- 16-month median DOR (range: 2 to 19+) versus 7 months (range: 2 to 19+).
Safety was assessed in 312 patients in the Libtayo combination group (median duration of exposure: 38 weeks) and 153 patients in the chemotherapy group (median duration of exposure: 21 weeks). The most common adverse reactions occurring in >15% of patients were alopecia (37% Libtayo combination, 43% placebo), musculoskeletal pain (30% Libtayo combination, 36% placebo), nausea (25% Libtayo combination, 16% placebo), fatigue (23% Libtayo combination, 18% placebo), peripheral neuropathy (23% Libtayo combination, 19% placebo) and decreased appetite (17% Libtayo combination, 12% placebo). Serious adverse reactions occurred in 25% of patients, with treatment discontinuations due to adverse reactions in 5% and fatal adverse reactions in 6%. No new Libtayo safety signals were observed.
“The approval of Libtayo in the combination setting builds on the monotherapy indication in advanced non-small cell lung cancer and furthers our excitement for what’s to come as we continue our potentially transformative oncology research,” said George D. Yancopoulos, M.D., Ph.D., President and Chief Scientific Officer at Regeneron. “Libtayo is the backbone of our oncology strategy, designed to synergistically combine multiple modalities to provide more options for more patients. We look forward to delivering on the promise of our research in other meaningful combinations that leverage Libtayo and our homegrown pipeline of investigational bispecific antibodies.”
“We welcome this latest approval for Libtayo as a first-line combination treatment for appropriate patients with advanced lung cancer,” said Andrea Ferris, President and CEO at the LUNGevity Foundation. “Lung cancer remains one of the most common cancers worldwide, and every new treatment option is an important step forward against this deadly cancer.”
About Non-small Cell Lung Cancer (NSCLC)
Lung cancer is the leading cause of cancer death worldwide. In recent years, more than 236,000 and 2.2 million annual new cases have been diagnosed in the U.S. and globally, respectively. Approximately 84% of all lung cancers are NSCLC, with 75% of these cases diagnosed in advanced stages. Additionally, 70% of all NSCLC cases will have <50% PD-L1 expression, making it the most common treatment setting.
About Regeneron’s VelocImmune Technology
Regeneron’s VelocImmune technology utilizes a proprietary genetically engineered mouse platform endowed with a genetically humanized immune system to produce optimized fully human antibodies. When Regeneron’s co-Founder, President and Chief Scientific Officer George D. Yancopoulos was a graduate student with his mentor Frederick W. Alt in 1985, they were the first to envision making such a genetically humanized mouse, and Regeneron has spent decades inventing and developing VelocImmune and related VelociSuite® technologies. Dr. Yancopoulos and his team have used VelocImmune technology to create approximately one in five of all original, FDA-approved or authorized fully human monoclonal antibodies. This includes REGEN-COV® (casirivimab and imdevimab), Dupixent® (dupilumab), Libtayo, Praluent® (alirocumab), Kevzara® (sarilumab), Evkeeza® (evinacumab-dgnb) and Inmazeb™ (atoltivimab, maftivimab and odesivimab-ebgn).
About Regeneron
Regeneron is a leading biotechnology company that invents, develops and commercializes life-transforming medicines for people with serious diseases. Founded and led for nearly 35 years by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to nine FDA-approved treatments and numerous product candidates in development, almost all of which were homegrown in our laboratories. Our medicines and pipeline are designed to help patients with eye diseases, allergic and inflammatory diseases, cancer, cardiovascular and metabolic diseases, pain, hematologic conditions, infectious diseases and rare diseases.
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules.
-
- Weblink: https://chemroboticspharma.com/pharmVetPat


