Summary:-
- TZIELD is the first disease-modifying therapy in T1D, a life-threatening autoimmune disease
- In a clinical trial, in Stage 2 T1D patients, TZIELD delayed the median onset of Stage 3 T1D by 25 months, or approximately 2 years, compared to placebo
- Stage 3 T1D is associated with significant health risks, including diabetic ketoacidosis, which can be life threatening
- Patients who progress to Stage 3 T1D eventually require insulin injections for life.
Provention Bio, Inc. (the “Company”), a biopharmaceutical company dedicated to intercepting and preventing immune-mediated diseases, today announced that the United States Food and Drug Administration (FDA) approved the Biologics License Application (BLA) for TZIELD (teplizumab-mzwv), an anti-CD3-directed antibody, for intravenous use, as the first and only immunomodulatory treatment to delay the onset of Stage 3 T1D in adult and pediatric patients aged 8 years and older with stage 2 T1D. The most common adverse reactions (>10%) that occurred during treatment and through 28 days after the last study drug administration from the TN-10 study were lymphopenia (73% TZIELD, 6% Placebo), rash (TZIELD 36%, Placebo 0%), leukopenia (TZIELD 21%, Placebo 0%) and headache (TZIELD 11%, Placebo 6%).
In October 2022 the company announced a co-promotion agreement for the U.S. launch of TZIELD for delay in onset of clinical T1D in at-risk individuals with Sanofi. Olivier Bogillot, Head of U.S. General Medicines, Sanofi, stated, “This approval is a profound and long-awaited victory for the diabetes community. We applaud Provention Bio for its unwavering determination to bring the first ever disease-modifying therapy for T1D to patients. We look forward to leveraging Sanofi’s established infrastructure and expertise in endocrinology to deliver for individuals in need across the U.S.”
Provention Bio has launched COMPASS, a patient support program with a staff of dedicated personnel available to answer questions and help navigate coverage, reimbursement and access for patients that are prescribed TZIELD.
About TZIELD
Structure of Teplizumab
- API– Teplizumab-mzwv
- Description– TZIELD (teplizumab-mzwv) is a CD3-directed antibody indicated to delay the onset of Stage 3 T1D in adults and pediatric patients aged 8 years and older with Stage 2 T1D. TZIELD injection is supplied as a sterile, preservative-free, clear and colorless solution in a 2 mg/2 mL (1 mg/mL) single-dose vial for intravenous use. TZIELD should be administered by intravenous infusion (over a minimum of 30 minutes) once daily for 14 days. Please see full prescribing information for the dosing schedule. If a patient needs help paying for TZIELD, Provention Bio’s Patient Assistance Program may be able to help. While co-pay amounts vary based on individual coverage, with the Provention Bio Copay Program, commercially or privately insured individuals enrolled in COMPASS may pay as little as $0 for TZIELD. If a patient qualifies, their COMPASS Navigator can help enroll them into the program so they may be able to lower their out-of-pocket costs.
- Class-Antihyperglycaemics; Monoclonal antibodies
- Mechanism of Action-CD3 antigen inhibitors
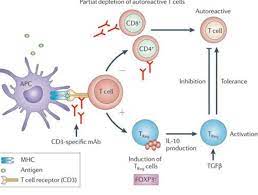
Teplizumab MOA
- Orphan Drug Status-Yes – Type 1 diabetes mellitus
- Indication–Type 1 diabetes mellitus
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: In clinical trials, lymphopenia occurred in 78% of TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines in TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment, or 6 weeks after completion of treatment.
‘Screening becomes a really big issue’
Aaron Kowalski, CEO of the Juvenile Diabetes Research Foundation, says the main challenge in prescribing Tzield will be finding people who need it. The drug is approved for people who don’t have any symptoms of the disease and may not know they’re on the road to getting it.
“Screening becomes a really big issue, because what we know is, about 85% of type 1 diagnoses today are in families that don’t have a known family history,” Kowalski said. “Our goal is to do general population screening” with blood tests to look for markers of the disease.
Tzield is approved for use in people 8 and older who are in stage 2 of their type 1 diabetes. In that stage, doctors can measure antibodies that attack insulin-producing beta cells in the person’s blood, and they have abnormal blood sugar levels, but their body can still make insulin.
“The way in which not just industry but our medical system go about managing autoimmune diseases, and especial type 1 diabetes, is really suboptimal in today’s day and age,” ProventionBio co-founder and CEO Ashleigh Palmer said. “What we do is, we wait until the symptoms of the disease present to doctors, and then doctors treat the patient’s symptoms chronically for a lifetime. The trouble is that in type 1 diabetes, when the symptoms first present, it’s too late.”
The treatment comes in a single 14-day course of infusions that each last 30 to 60 minutes.
The most common side effects reported in the trial participants were low white blood cells and lymph cells, rash and headache.
The potential to help millions
A delayed diagnosis of type 1 diabetes could have a significant impact.
“Obviously, the quality of life is substantially impacted, negatively impacted, if you are diagnosed with type 1 diabetes. It’s a disease that never goes away,” Palmer said.
People who are type 1 diabetics must monitor their blood glucose levels around the clock, affecting how they exercise and eat. High blood sugar can lead to diabetic ketoacidosis, in which the body starts to break down fat as its fuel, and can cause a buildup of acids called ketones in the bloodstream. That condition can lead to hospitalization, coma or death.
As of 2019, about 1.9 million people have type 1 diabetes in the United States, according to the American Diabetes Association, including 244,000 children and adolescents. Type 1 affects 8% of everyone with diabetes.
The Unmet Need in T1D
Over 1.8 million Americans have T1D, an autoimmune disease caused by the destruction of beta cells. Diagnosis of T1D usually occurs in children and young adults, but it can happen at any age after symptoms appear when a person cannot make enough insulin. However, T1D starts in the body long before any symptoms appear and can be detected at this pre-symptomatic stage through a blood test. The psychological impact of T1D is hard to quantify, but a diagnosis is life-altering, and regular monitoring and maintenance can be extremely stressful. T1D typically takes more than a decade off a person’s life, and life expectancy is reduced by 16 years on average for people diagnosed before the age of 10. Insulin therapy and glucose monitoring are currently the standard of care for treating clinical-stage, or Stage 3 T1D, and are necessary to keep T1D patients alive. The constant monitoring and administration of insulin represents a significant life-long burden for patients.
TN-10 Study
TZIELD was investigated in the TN-10 Study, a pivotal randomized, double-blind, event driven, placebo controlled clinical trial which evaluated TZIELD for the delay of T1D (Stage 3, or clinical T1D) in Stage 2 T1D patients, defined by the presence of two or more T1D-related autoantibodies and dysglycemia. Seventy-six patients (TZIELD N=44, placebo N=32) were enrolled ages 8 to 49, with 72% under the age of 18, and randomized to receive a single 14-day course of either teplizumab or placebo by IV infusion. The primary efficacy endpoint in this study was the time from randomization to development of Stage 3 T1D diagnosis.
In Study TN-10, Stage 3 T1D was diagnosed in 20 (45%) of the TZIELD-treated patients and in 23 (72%) of the placebo-treated patients. A Cox proportional hazards model was used to analyze the time to Stage 3 T1D diagnosis, stratified by age and oral glucose tolerance test status at randomization. The median time from randomization to Stage 3 T1D diagnosis was 50 months in the TZIELD group and 25 months in the placebo group, for a difference of 25 months. With a median follow-up time of 51 months, therapy with TZIELD resulted in a statistically significant delay in the development of Stage 3 T1D, hazard ratio 0.41 (95% CI: 0.22 to 0.78; p=0.0066).
The most common adverse reactions (>10%) that occurred during treatment and through 28 days after the last study drug administration from the TN-10 study were lymphopenia (73% TZIELD, 6% Placebo), rash (TZIELD 36%, Placebo 0%), leukopenia (TZIELD 21%, Placebo 0%) and headache (TZIELD 11%, Placebo 6%).
About Sanofi US Co-Promotion
In October 2022, Provention entered into a co-promotion agreement with Sanofi U.S. for the launch of TZIELD. Under the terms of the agreement, Sanofi will commit commercial resources in the United States, including diabetes field specialists, account directors, field-based reimbursement, and medical science liaisons to expand the number of key healthcare professionals reached in the United States. In exchange, Provention will reimburse field force-related expenses that Sanofi will incur in connection with commercializing teplizumab under the agreement.
Provention retains all rights to TZIELD and maintains responsibility for the commercialization strategy.
About Provention Bio, Inc.
Provention Bio, Inc is a biopharmaceutical company focused on advancing the development of investigational therapies that may intercept and prevent debilitating and life-threatening immune-mediated diseases. The Company’s pipeline includes clinical-stage product candidates that have demonstrated in pre-clinical or clinical studies proof-of-mechanism and/or proof-of-concept in autoimmune diseases, including T1D, celiac disease and lupus.
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules.
-
- Weblink: https://chemroboticspharma.com/pharmVetPat


