Synopsis :
- Opzelura is the first and only FDA-approved product for repigmentation in nonsegmental vitiligo
- Phase 3 data supporting the approval show treatment with Opzelura resulted in improvements in facial and total body repigmentation
- Fifty-two week data demonstrate continued improvements in repigmentation with longer duration of treatment
Incyte announced that the U.S. Food and Drug Administration (FDA) has approved Opzelura™ (ruxolitinib) cream 1.5% for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. Opzelura is the first and only FDA-approved treatment for repigmentation in patients with vitiligo, and the only topical formulation of a Janus kinase (JAK) inhibitor approved in the United States. Vitiligo is a chronic autoimmune disease characterized by depigmentation of skin.
About Opzelura™ (Ruxolitinib)
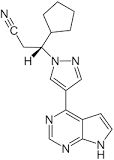
Ruxolitinib Structure
- API -Ruxolitinib
- Description – Ruxolitinib is in a class of medications called kinase inhibitors. It works to treat myelofibrosis and PV by blocking the signals that cause cancer cells to multiply. This helps to stop the spread of cancer cells. It works to treat GVHD by blocking the signals of the cells that cause GVHD.Opzelura, a novel cream formulation of Incyte’s selective JAK1/JAK2 inhibitor ruxolitinib, is the first and only topical JAK inhibitor approved for use in the United States, indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older and the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis (AD) in non-immunocompromised patients 12 years of age and older whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable. Use of Opzelura in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants, such as azathioprine or cyclosporine, is not recommended.
- Class- 2 ring heterocyclic compounds; Anti-inflammatories; Antianaemics; Antibronchitics; Antihaemorrhagics; Antineoplastics; Antipsoriatics; Antirheumatics; Antivirals; Cyclopentanes; Nitriles; Pyrazoles; Pyrimidines; Pyrroles; Skin disorder therapies; Small molecules
- Mechanism of Action –Janus kinase 1 inhibitors; Janus kinase-2 inhibitors
- Orphan Drug Status-Yes – Myelofibrosis; Pancreatic cancer; Polycythaemia vera; Essential thrombocythaemia; Precursor cell lymphoblastic leukaemia-lymphoma; Graft-versus-host disease
OPZELURA may cause serious side effects, including:
Serious Infections: OPZELURA contains ruxolitinib. Ruxolitinib belongs to a class of medicines called Janus kinase (JAK) inhibitors. JAK inhibitors are medicines that affect your immune system. JAK inhibitors can lower the ability of your immune system to fight infections. Some people have had serious infections while taking JAK inhibitors by mouth, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have been hospitalized or died from these infections. Some people have had serious infections of their lungs while taking OPZELURA. Your healthcare provider should watch you closely for signs and symptoms of TB during treatment with OPZELURA.
Increased risk of death due to any reason (all causes): Increased risk of death has happened in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and are taking a medicine in the class of medicines called JAK inhibitors by mouth.
Cancer and immune system problems: OPZELURA may increase your risk of certain cancers by changing the way your immune system works. Lymphoma and other cancers have happened in people taking a medicine in the class of medicines called JAK inhibitors by mouth. People taking JAK inhibitors by mouth have a higher risk of certain cancers including lymphoma and lung cancer, especially if they are a current or past smoker. Some people have had skin cancers while using OPZELURA. Your healthcare provider will regularly check your skin during your treatment with OPZELURA. Limit the amount of time you spend in the sunlight. Wear protective clothing when you are in the sun and use a broad-spectrum sunscreen.
Increased risk of major cardiovascular events: Increased risk of major cardiovascular events such as heart attack, stroke, or death have happened in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and taking a medicine in the class of medicines called JAK inhibitors by mouth, especially in current or past smokers.
Blood clots: Blood clots in the veins of your legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) can happen in some people taking OPZELURA. This may be life-threatening. Blood clots in the vein of the legs (deep vein thrombosis, DVT) and lungs (pulmonary embolism, PE) have happened more often in people who are 50 years of age and older and with at least 1 heart disease (cardiovascular) risk factor taking a medicine in the class of medicines called JAK inhibitors by mouth.
Low blood cell counts: OPZELURA may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia), and low white blood cell counts (neutropenia). If needed, your healthcare provider will do a blood test to check your blood cell counts during your treatment with OPZELURA and may stop your treatment if signs or symptoms of low blood cell counts happen.
Cholesterol increases: Cholesterol increase has happened in people when ruxolitinib is taken by mouth. Tell your healthcare provider if you have high cholesterol or triglycerides.
“With the approval of Opzelura in nonsegmental vitiligo, Incyte has once again delivered a treatment to patients with high unmet medical need who previously had no approved therapies,” said Hervé Hoppenot, Chief Executive Officer, Incyte. “We are proud of Incyte’s scientists and development teams that have made this milestone possible, and we’re pleased that eligible vitiligo patients now have a choice to address repigmentation.”
“It’s very exciting and quite an honor to be able to bring the first treatment option for these patients,” Lee said.
In patients with non-segmental vitiligo, Opzelura is approved for continuous topical use twice daily to affected areas of up to 10% body surface area. Satisfactory patient response may require treatment with Opzelura for more than 24 weeks.
The FDA approval was based on data from the pivotal Phase 3 TRuE-V clinical trial program (TRuE-V1 and TRuE-V2), evaluating the safety and efficacy of Opzelura versus vehicle in more than 600 people with nonsegmental vitiligo, age 12 and older. In the studies, treatment with Opzelura resulted in significant improvements in VASI scores, which represent improvements in facial and total body repigmentation at Week 24 (primary analysis) compared to vehicle (non-medicated cream) and in an open-label extension at Week 52.
Additionally, at Week 24, more than 15% of patients treated with Opzelura achieved ≥90% improvement from baseline in F-VASI (F-VASI90), compared to approximately 2% of patients treated with vehicle. At Week 52, the percentage of Opzelura-treated patients who achieved F-VASI90 doubled to approximately 30%.
In the vehicle controlled period of the Phase 3 studies, the most common adverse reactions (incidence ≥ 1%) are application site acne, application site pruritus, nasopharyngitis, headache, urinary tract infection, application site erythema, and pyrexia. The labeling for Opzelura includes a Boxed Warning for serious infections, mortality, malignancy, major adverse cardiovascular events and thrombosis.
About Vitiligo
Vitiligo is a chronic autoimmune disease characterized by depigmentation of skin that results from the loss of pigment-producing cells known as melanocytes. Over-activity of the JAK signaling pathway is believed to drive inflammation involved in the pathogenesis and progression of vitiligo. In the United States, more than 1.5 million people are diagnosed with vitiligo. The overall prevalence of the condition is estimated to be approximately 2-3 million, with the majority of patients (approximately 85%) suffering from nonsegmental vitiligo. Vitiligo can occur at any age, although many patients with vitiligo will experience initial symptoms before the age of 30.
About Incyte Dermatology
Incyte’s science-first approach and expertise in immunology has formed the foundation of the company. In Dermatology, the Company’s research and development efforts are focused on leveraging our knowledge of the JAK-STAT pathway to identify and develop topical and oral therapies with the potential to modulate immune pathways driving uncontrolled inflammation and help restore normal immune function.
Currently, Incyte is exploring the potential of JAK inhibition for additional immune-mediated dermatologic conditions with a high unmet medical need, including hidradenitis suppurativa.
About Incyte
Incyte is a Wilmington, Delaware-based, global biopharmaceutical company focused on finding solutions for serious unmet medical needs through the discovery, development and commercialization of proprietary therapeutics.
Weblink: https://www.chemrobotics.com
- AgroPat Lite– Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. Covers the product information including formulation, combination, developer, innovator, existing intellectual property, regulatory requirement, biology data including spectrum, MOA, DFU, toxicity profile, and safety. (Designed for Business Development function)
-
-
- AgroPat Ultimate– In detailed Access 5500 pesticides with chemistry, Biology, Regulatory, and IP info. (Designed for Research & Development function)
- Indian Medicine Database –Approved Drugs, Medical Devices, Approved Regenerative Medical Products
- Weblink: https://imd.chemrobotics.com/
- Indian Pesticide Database (IPD)– All Indian Approvals, e.g. 9(3) and 9(4), etc.
- Global Agro Product Directory(More than 55countries approved product info. with relevant documents such as label, factsheet and monograph)
- Weblink: https://www.chemrobotics.com/pesticides-directory/
- Global MRL Database(More than 85 countries MRL info.)
- Jarvis– A Competitor Patents Watch Database for Agrochemical
- Technical Routes(More than 15000 routes of synthesis for Agrochemical & Pharmaceutical)
- Technical Suppliers(Provides technical supplier information)
- Weblink: https://www.chemrobotics.com/agrochem/technical-supplier
- Company Directory– KSM Supplier(s) Database — More than 10 K Companies listed from Pharma / Agrochemical / Fine Chemical Domain with their product offering in Pharma / Agrochemical / Fine Chemical segment,
- Weblink: https://companydirectory.chemrobotics.com
- ChemRobotics SPC Database– Provides Patent SPC data Europe
- Weblink: https://chemrobotics.com/agropharm/#/spcdb
- PharmVetPat –Access chemistry including ROS, KSM, Intermediate, Biology, Regulatory, and IP info for all pharm molecules
- Weblink: https://chemroboticspharma.com/pharmVetPat
-


