Synopsis-
Sun Pharmaceuticals Industries announced that one of its wholly-owned subsidiaries has received final approval from US FDA for its Abbreviated New Drug Application (ANDA) for generic Amphotericin B Liposome for Injection, 50 mg/vial Single-Dose Vial.
The generic product approval is based on AmBisome Liposome for Injection, 50 mg/vial as a reference product.
Sun Pharma has been granted Competitive Generic Therapy (CGT) designation by US FDA and being the first approved generic, is eligible for 180 days of CGT exclusivity for the product.

USFDA Approves Generic Amphotericin B Liposome for Injection Manufactured By Sun Pharma
As per October 2021 IQVIA Health data, AmBisome Liposome for Injection, 50mg/vial had annualized sales of approximately US$ 136 million in USA.
Shares of Sun Pharmaceutical Industries Limited was last trading in BSE at Rs. 775.55 as compared to the previous close of Rs. 755.95. The total number of shares traded during the day was 359587 in over 6149 trades.
The stock hit an intraday high of Rs. 778.75 and intraday low of 745.45. The net turnover during the day was Rs. 272721437.00.
About Amphotericin B Liposome
Liposomal amphotericin B is a lipid-associated formulation of the broad-spectrum polyene antifungal agent amphotericin B.
It is active against clinically relevant yeasts and moulds, including Candida spp., Aspergillus spp. and filamentous moulds such as Zygomycetes, and is approved for the treatment of invasive fungal infections in many countries worldwide. It was developed to improve the tolerability profile of amphotericin B deoxycholate, which was for many decades considered the gold standard of antifungal treatment, despite being associated with infusion-related events and nephrotoxicity. In well controlled trials, liposomal amphotericin B had similar efficacy to amphotericin B deoxycholate and amphotericin B lipid complex as empirical therapy in adult and paediatric patients with febrile neutropenia. In addition, caspofungin was noninferior to liposomal amphotericin B as empirical therapy in adult patients with febrile neutropenia. For the treatment of confirmed invasive fungal infections, liposomal amphotericin B was more effective than amphotericin B deoxycholate treatment in patients with disseminated histoplasmosis and AIDS, and was noninferior to amphotericin B deoxycholate in patients with acute cryptococcal meningitis and AIDS. In adults, micafungin was shown to be noninferior to liposomal amphotericin B for the treatment of candidaemia and invasive candidiasis. Data from animal studies suggested that higher dosages of liposomal amphotericin B might improve efficacy; however, in the AmBiLoad trial in patients with invasive mould infection, there was no statistical difference in efficacy between the standard dosage of liposomal amphotericin B 3 mg/kg/day and a higher 10 mg/kg/day dosage, although the standard dosage was better tolerated.
Structure
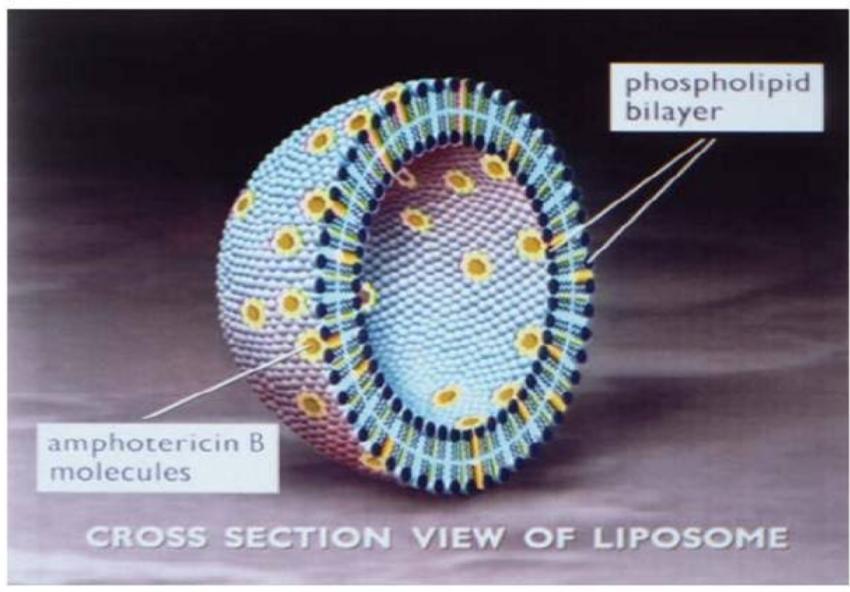
Structure Amphotericin B Liposome
Despite being associated with fewer infusion-related adverse events and less nephrotoxicity than amphotericin B deoxycholate and amphotericin B lipid complex, liposomal amphotericin B use is still limited to some extent by these adverse events. Both echinocandins were better tolerated than liposomal amphotericin B. The cost of liposomal amphotericin B therapy may also restrict its use, but further pharmacoeconomic studies are required to fully define its cost effectiveness compared with other antifungal agents. Based on comparative data from well controlled trials, extensive clinical experience and its broad spectrum of activity, liposomal amphotericin B remains a first-line option for empirical therapy in patients with febrile neutropenia and in those with disseminated histoplasmosis, and is an option for the treatment of AIDS-associated cryptococcal meningitis, and for invasive Candida spp. or Aspergillus spp. infections. Amphotericin B, a macrocyclic, polyene antifungal agent, is thought to act by binding to ergosterol, the principal sterol in fungal cell membranes and Leishmania cells. This results in a change in membrane permeability, causing metabolic disturbance, leakage of small molecules and, as a consequence, cell death. In vitro and in vivo studies have shown that liposomal amphotericin B remains closely associated with the liposomes in the circulation, thereby reducing the potential for nephrotoxicity and infusion-related toxicity associated with conventional amphotericin B. Amphotericin B shows very good in vitro activity against a broad spectrum of clinically relevant fungal isolates, including most strains of Candida spp. and Aspergillus spp., and other filamentous fungi such as Zygomycetes. Liposomal amphotericin B has proven effective in various animal models of fungal infections, including those for candidiasis, aspergillosis, fusariosis and zygomycosis. Liposomal amphotericin B also shows immunomodulatory effects, although the mechanisms involved are not fully understood, and differ from those of amphotericin B deoxycholate and amphotericin B colloidal dispersion. In adult patients with febrile neutropenia, intravenous liposomal amphotericin B has nonlinear pharmacokinetics, with higher than dose-proportional increases in exposure being consistent with reticuloendothelial saturation and redistribution of amphotericin B in the plasma compartment. Liposomal amphotericin B is rapidly and extensively distributed after single and multiple doses, with steady-state concentrations of amphotericin B attained within 4 days and no clinically relevant accumulation of the drug following multiple doses of 1-7.5 mg/kg/day. In autopsy tissue, the highest concentrations of the drug were found in the liver and spleen, followed by the kidney, lung, myocardium and brain tissue. Elimination of liposomal amphotericin B, like that of amphotericin B deoxycholate, is poorly understood; its route of metabolism is not known and its excretion has not been studied. The terminal elimination half-life is about 7 hours. No dosage adjustment is required based on age or renal impairment. In several randomized, double-blind trials (n = 73-1095) in adult and/or paediatric patients, liposomal amphotericin B was effective as empirical therapy or as treatment for confirmed invasive fungal infections, including invasive candidiasis, candidaemia, invasive mould infection (mainly aspergillosis), histoplasmosis and cryptococcal meningitis. All agents were administered as an intravenous infusion; the typical dosage for liposomal amphotericin B was 3 mg/kg/day. Treatment was generally given for 1-2 weeks. Participants in trials evaluating empirical therapy had neutropenia and a persistent fever despite antibacterial treatment and had received chemotherapy or undergone haematopoietic stem cell transplantation. As empirical therapy in adult and paediatric patients, liposomal amphotericin B appeared to be as effective as amphotericin B deoxycholate (approximately 50% of patients in each group achieved treatment success) or amphotericin B lipid complex (approximately 40% of liposomal amphotericin B recipients experienced treatment success).
Why is this medication prescribed?
Amphotericin B liposomal injection is used to treat fungal infections such as cryptococcal meningitis (a fungal infection of the lining of the spinal cord and brain) and visceral leishmaniasis (a parasitic disease that usually affects spleen, liver, and bone marrow) in certain people. It is also used to treat certain fungal infections in people who cannot receive conventional amphotericin B therapy. Amphotericin B liposomal injection is in a class of medications called antifungals. It works by slowing the growth of fungi that cause infection.
Amphotericin B Liposome is used to treat various Fungal Infections
How should this medicine be used?
Amphotericin B liposomal injection comes as a suspension (liquid) to be injected intravenously (into a vein). It is usually infused intravenously once daily, or for the treatment of leishmaniasis on specific days, over a period of 2 hours. If previous doses are tolerated, this medication may be given over a period of 1 hour. The length of your treatment depends on your general health, how you tolerate the medication, and the type of infection you have.
You may experience a reaction while you receive a dose of amphotericin B liposomal complex injection. These reactions usually happen 1 to 3 hours after starting your infusion and are more severe with the first few doses. Your health care provider may prescribe other medications to decrease these side effects. Tell your doctor immediately if you experience any of these symptoms while you receive amphotericin B liposomal complex injection: fever, chills, nausea, vomiting, flushing, back pain with or without chest tightness, chest pain, shortness of breath, breathing problems, or a fast, irregular, or pounding heartbeat.
You may receive amphotericin B liposomal injection in a hospital or you may use the medication at home. If you will be using amphotericin B liposomal injection at home, your healthcare provider will show you how to infuse the medication. Be sure that you understand these directions, and ask your healthcare provider if you have any questions. Ask your healthcare provider what to do if you have any problems infusing amphotericin B liposomal injection.
If your symptoms do not improve or get worse while receiving amphotericin B liposomal injection, tell your doctor. If you still have symptoms of infection after you finish amphotericin B liposomal injection, tell your doctor.
Mechanism of Action
Amphotericin B binds to ergosterol in the fungal cell membrane, which leads to the formation of pores, ion leakage and ultimately fungal cell death.
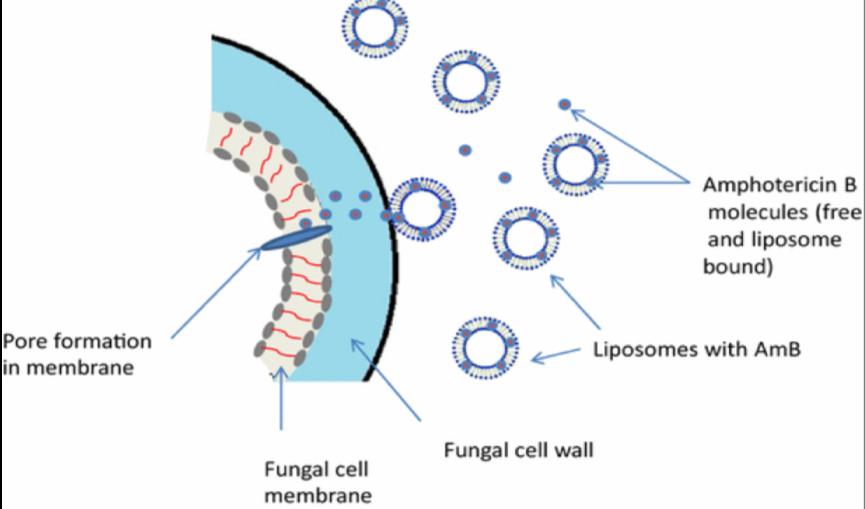
MOA of Amphotericin B Liposome
Amphotericin B is a polyene antifungal that exerts its activity by binding to ergosterol in fungal cell membranes, developing holes in the membrane and allowing cell components to leak out, causing cell death
For more Information: Sign in Websites for Agrochemical & Pharmaceutical Databases:
Website : https://www.chemrobotics.com/ (Agrochemical Databases)
Website : https://chemroboticspharma.com/ (Pharmaceutical Databases)

