Synopsis
-Ahead of its New Year’s Day decision deadline at the FDA, Xeris Biopharma has snagged an approval for Recorlev, a drug formerly known as levoketoconazole.
Based on results from phase 3 studies called SONICS and LOGICS, the FDA approved the drug for adults with Cushing’s syndrome. Xeris picked up Recorlev earlier this year in its acquisition of rare disease biotech Strongbridge Biopharma. It’s planning to launch in the first quarter of 2022.
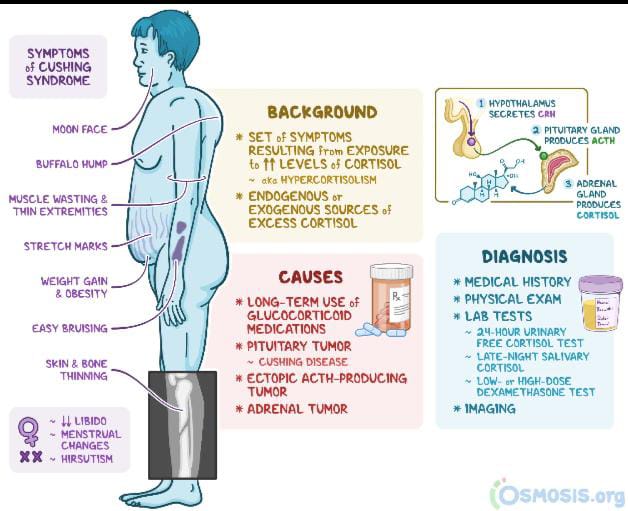
Recorlev’s approval covers the treatment of endogenous hypercortisolemia in adults with Cushing’s syndrome who aren’t eligible for surgery or haven’t responded to surgery.
Endogenous Cushing’s disease is caused by a benign tumor in the pituitary gland that prompts the body to produce elevated levels of cortisol, which over time triggers a range of devastating physical and emotional symptoms for patients.
In the SONICS study, the drug significantly cut and normalized mean urinary free cortisol concentrations without a dose increase, according to the company. The LOGICS trial confirmed the drug’s efficacy and safety, Xeris says.

Xeris Biopharma scores FDA approval for endogenous Cushing’s syndrome drug Recorlev
Cushing’s is a potentially fatal endocrine disease, and patients often experience years of symptoms before an accurate diagnosis, the company says. After a diagnosis, they’re presented with limited effective treatment options.
Following the approval, the company’s “experienced endocrinology-focused commercial organization can begin rapidly working to help address the needs of Cushing’s syndrome patients in the U.S. who are treated with prescription therapy,” Xeris CEO Paul R. Edick said in a statement.
Aside from its forthcoming Recorlev launch, Xeris markets Gvoke for severe hypoglycemia and Keveyis for primary periodic paralysis.
Back in October, the company partnered up with Merck to help reformulate some of the New Jersey pharma giant’s monoclonal antibody drugs.
About Cushing Syndrome